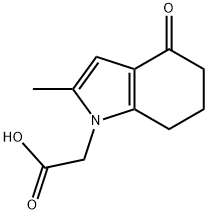
2-(2-METHYL-4-OXO-4,5,6,7-TETRAHYDRO-1H-INDOL-1-YL)ACETIC ACID synthesis
- Product Name:2-(2-METHYL-4-OXO-4,5,6,7-TETRAHYDRO-1H-INDOL-1-YL)ACETIC ACID
- CAS Number:1260673-37-7
- Molecular formula:C11H13NO3
- Molecular Weight:207.23
Yield:1260673-37-7 30%
Reaction Conditions:
with acetic acid for 1.5 h;Inert atmosphere;Reflux;
Steps:
3A
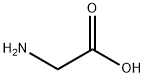
A stirred mixture of 2-(2-oxopropyl)cyclohexane- 1 ,3-dione (prepared by evaporation of an ethanolic solution to dryness under vacuum) (20.Og, 119mmol) and glycine (17.9g, 238mmol) in acetic acid (10OmL) was heated under reflux for 1.5h then allowed to cool to r.t. Water (10OmL) was then added and the mixture evaporated to a thick oil in vacuo. Acetone (20OmL) and water (4OmL) were added to the residue and the mixture stirred for 30mins at r.t., after which the solid was collected by filtration and the filtrates retained.The solid was slurried with further acetone (10OmL) and water (2OmL) at r.t. then removed by filtration. The combined filtrates were concentrated in vacuo then dissolved in aqueous sodium hydroxide (IM, 20OmL), adding a small amount of 1OM sodium hydroxide to bring the pH to 14. After washing with ethyl acetate (2 x 10OmL), the mixture was acidified by the addition of aqueous hydrochloric acid (5M, 6OmL) then sodium chloride (5Og) was added. After stirring for 4h, the solid product was collected by filtration, washed with acetone (2 x 25mL) then dried under vacuum at 4O0C to afford the title compound as an orange-brown solid; 7.45g (30%); purity 99.1 area% by HPLC; m/z: 208 (MH+); 1H1 NMR: 13.2 (IH, br s), 6.02 (IH, s), 4.67 (2H, s), 2.65 (2H, t, J = 6.2Hz), 2.29 - 2.24 (2H, m), 2.10 (3H, s), 2.02 - 1.95 (2H, m).
References:
WO2011/4182,2011,A1 Location in patent:Page/Page column 38

504-02-9
553 suppliers
$5.00/10g

1260673-37-7
8 suppliers
inquiry
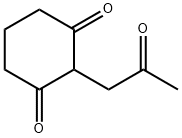
90534-62-6
2 suppliers
inquiry

1260673-37-7
8 suppliers
inquiry