
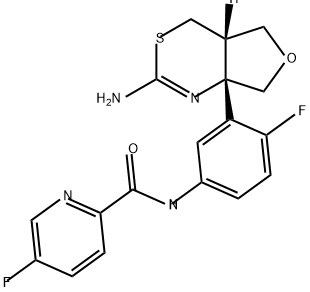
N-[3-[(4aS,7aS)-2-Amino-5,7-dihydro-4H-furo[3,4-d][1,3]thiazin-7a(4aH)-yl]-4-fluorophenyl]-5-fluoro-2-pyridinecarboxamide hydrochloride synthesis
- Product Name:N-[3-[(4aS,7aS)-2-Amino-5,7-dihydro-4H-furo[3,4-d][1,3]thiazin-7a(4aH)-yl]-4-fluorophenyl]-5-fluoro-2-pyridinecarboxamide hydrochloride
- CAS Number:1262036-49-6
- Molecular formula:C18H16F2N4O2S.HCl
- Molecular Weight:426.868
1262036-50-9
118 suppliers
$43.00/1mg
![N-[3-[(4aS,7aS)-2-Amino-5,7-dihydro-4H-furo[3,4-d][1,3]thiazin-7a(4aH)-yl]-4-fluorophenyl]-5-fluoro-2-pyridinecarboxamide hydrochloride](/CAS/GIF/1262036-49-6.gif)
1262036-49-6
29 suppliers
inquiry
Yield:1262036-49-6 94%
Reaction Conditions:
with hydrogenchloride in 1,4-dioxane;ethanol;dichloromethane at 22; for 1 h;
Steps:
1a
To a suspension of N-(3-((4aS,7aS)-2-amino-4a,5,7,7a-tetrahydro-4H-furo[3,4-d][1,3]thiazin-7a-yl)-4-fluorophenyl)-5-fluoropicolinamide (17.3 g, 42.10 mmol) in a mixture of ethanol (631.45 mL) and dichloromethane (420.96 mL) is added a solution of 4 M hydrogen chloride in 1,4-dioxane (46.31 mL, 185.22 mmol). The mixture is stirred at 22° C. for 1 h, concentrated and the residue is triturated with EtOH (200 mL). The solid is filtered off and washed with EtOH. The solid is triturated with water and the suspension is concentrated under reduced pressure. The residue is dried in a vacuum oven (18 h, 40° C.) to give the title compound as a white solid (17.1 g, 94%). ES/MS m/e: 391 (M+1).
References:
US2011/9395,2011,A1 Location in patent:Page/Page column 33
![Carbamic acid, N-[(4aS,7aS)-7a-[2-fluoro-5-[[(5-fluoro-2-pyridinyl)carbonyl]amino]phenyl]-4a,5,7,7a-tetrahydro-4H-furo[3,4-d][1,3]thiazin-2-yl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/1262038-80-1.gif)
1262038-80-1
0 suppliers
inquiry
![N-[3-[(4aS,7aS)-2-Amino-5,7-dihydro-4H-furo[3,4-d][1,3]thiazin-7a(4aH)-yl]-4-fluorophenyl]-5-fluoro-2-pyridinecarboxamide hydrochloride](/CAS/GIF/1262036-49-6.gif)
1262036-49-6
29 suppliers
inquiry
107504-08-5
262 suppliers
$7.00/250mg
![N-[3-[(4aS,7aS)-2-Amino-5,7-dihydro-4H-furo[3,4-d][1,3]thiazin-7a(4aH)-yl]-4-fluorophenyl]-5-fluoro-2-pyridinecarboxamide hydrochloride](/CAS/GIF/1262036-49-6.gif)
1262036-49-6
29 suppliers
inquiry