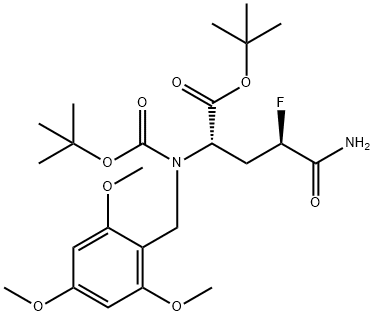
1262523-70-5 synthesis
- Product Name:1262523-70-5
- CAS Number:1262523-70-5
- Molecular formula:C24H37FN2O8
- Molecular Weight:500.56
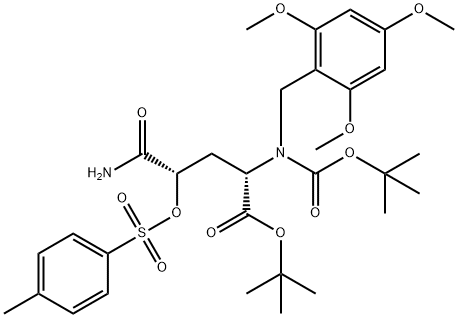
![L-Glutamine, N2-[(1,1-dimethylethoxy)carbonyl]-4-[[(4-methylphenyl)sulfonyl]oxy]-N-[(2,4,6-trimethoxyphenyl)methyl]-, 1,1-dimethylethyl ester, (4R)-](/CAS/20210305/GIF/1262523-68-1.gif)
1262523-68-1
0 suppliers
inquiry

1262523-70-5
3 suppliers
inquiry
Yield:1262523-70-5 77%
Reaction Conditions:
with tris(dimethylamino)sulfonium trimethylsilyldifluoride;triethylamine in tetrahydrofuran;dichloromethane at 50; for 10 h;
Steps:
To a stirred solution of tris(dimethylamino)sulfonium difluorotrimethylsilicate (TASF, 1.38 g, 5.0 mmol) in CH2C12/THF (1.5 mL/1.5 mL) was added Et3ND(HF)3 (0.25 mL) dropwise. After that, tosylate 11' (0.391 g, 0.6 mmol), THF (1 mL) and above "neutralized" TASF (2.7 mL, 3.0 mmol) were added one by one to a 25 mL two-neck flask equipped with a reflux condenser. The mixture was heated to 50 0C by oil bath for 1O h, the oil bath was removed, the reaction mixture was diluted with EtOAc and washed with half saturated NaHCO3, water and brine subsequently. The EtOAc phase was collected, dried by MgSO4, filtered, concentrated in vacuo. The left residue was purified by FC (EtOAc/Hexanes, 25/75 to 40/60, V/V) to provide a pale white foamy solid 12' (0.232 g, 77%); [α]24D = + 1.70 (c = 1.14, MeOH); HPLC of 12': [99%, major peak: Rt = 22.8 min; 1%, minor peak: Rt = 25.2 min; column: Chiralcel OD (250 x 4.6 mm), UV detector, 210 nm, 1.5% EtOH in hexanes; flow rate: 1.2 mL/min]. 1H NMR (200 MHz, CDCl3) δ 6.69 (br s, 1 H), 6.12 (s, 2 H), 5.32 (d, 1 H, J= 8.6 Hz), 5.13 (dd, 0.5 H, J1 = 8.8 Hz, J2 = 3.2 Hz), 4.88 (dd, 0.5 H, J1 = 8.8 Hz, J2 = 3.2 Hz), 4.60 (dd, 1 H, J1 = 13.6 Hz, J2 = 6.0 Hz), 4.42- 4.34 (m, 2 H), 3.81 (s, 9 H), 2.64-2.06 (m, 2 H), 1.45 (s, 9 H), 1.43 (s, 9 H). 13C NMR (50 MHz, CDCl3) £170.9, 168.8, 168.4, 161.3, 159.5, 155.3, 106.2, 91.2, 90.8, 87.5, 82.5, 80.0, 56.0, 55.6, 51.2, 35.7, 35.3, 32.2, 28.5, 28.1. HRMS calcd for C24H38FN2O8 (M + H)+: 501.2612; found:501.2613.Crystals of 12' suitable for X-ray crystal structure analysis were obtained from a slow solvent evaporation of CH2C12/Hexanes solution of 12'.
References:
WO2011/20018,2011,A1 Location in patent:Page/Page column 29-30
1262523-66-9
7 suppliers
inquiry

1262523-70-5
3 suppliers
inquiry

480438-26-4
2 suppliers
inquiry

1262523-70-5
3 suppliers
inquiry
![L-Glutamine, 4-[(2-chloroacetyl)oxy]-N2-[(1,1-dimethylethoxy)carbonyl]-N-[(2,4,6-trimethoxyphenyl)methyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/1262523-46-5.gif)
1262523-46-5
1 suppliers
inquiry

1262523-70-5
3 suppliers
inquiry

1262523-62-5
3 suppliers
inquiry

1262523-70-5
3 suppliers
inquiry