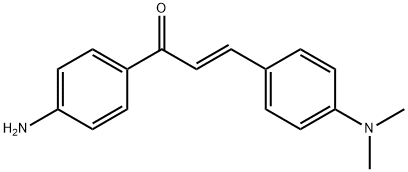
(2E)-1-(4-aminophenyl)-3-[4-(dimethylamino)phenyl]prop-2-en-1-one synthesis
- Product Name:(2E)-1-(4-aminophenyl)-3-[4-(dimethylamino)phenyl]prop-2-en-1-one
- CAS Number:126443-09-2
- Molecular formula:C17H18N2O
- Molecular Weight:266.34
4-aminoacetophenone( 1 g, 6.7 mmol) was suspended in ethanol (20 mL), to the suspension 40% of NaOH and 4-dimethylaminobenzaldeyde(0.904 g, 6.7 mmol) were added and stir this reaction mixture at RT for 16h. After completion of the reaction, the reaction mixture was poured into the 100 mL of ice water and P H is adjusted to 1 by drop wise adding of dil. HCl. The reaction mixture was filtered; the filtrate was neutralized with 5% NaHCO3 and subsequently extracted with CH2Cl2. The organic fraction was concentrated in vacuum under reduced pressure. Yield 1.569 g (86%).
1H NMR (400 MHz, CDCl3) δ, 8.10 (dd, J = 15.6, 8.5 Hz, 2H), 7.98 – 7.69 (m, 1H), 7.59 (dd, J = 8.8, 1.9 Hz, 1H), 7.42 (ddd, J = 15.4, 14.6, 6.7 Hz, 2H), 7.20 – 6.89 (m, 2H), 6.75 (dd, J = 15.5, 8.8 Hz, 2H), 3.26 – 2.91 (m, 6H), 1.64 (s, 2H), 13C NMR (101 MHz, CDCl3) δ 161.34 – 161.14 (m), 130.85 (s), 129.58 (s), 121.45 – 121.25 (m), 111.86 (s), 77.35 (s), 77.03 (s), 76.71 (s), 40.17 (s).
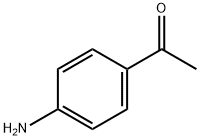
99-92-3
507 suppliers
$6.00/10g
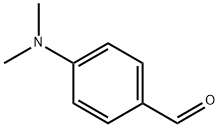
100-10-7
602 suppliers
$5.00/1g
![(2E)-1-(4-aminophenyl)-3-[4-(dimethylamino)phenyl]prop-2-en-1-one](/CAS/20140525/GIF/CB02131373.gif)
126443-09-2
9 suppliers
$126.00/500mg
Yield:126443-09-2 87%
Reaction Conditions:
with sodium hydroxide in ethanol;Microwave irradiation;
Steps:
2.2. Synthesis of amino chalcones
General procedure: The preparation of amino chalcones was accomplished by microwave assisted synthesis [22]. An equimolar ratio of 4-aminoacetophenone (1 mmol) and aromatic aldehyde (1 mmol) were dissolved in 95% ethanol with catalytic quantity of NaOH (1-2 pellets). Further, the reaction mixture was irradiated under 180 microwave radiations for 10-15 mins and the progress of the reaction was confirmed with TLC using n-hexane-ethyl acetate (7:3). Subsequently, the reaction mixture was cooled at room temperature and poured into ice water. The solid was separated, filtered and recrystallized using ethanol (neutralize with dil. HCl, if precipitate is not appeared).
References:
Bhaskar, Baki Vijaya;Gu, Wei;Rammohan, Aluru;Venkateswarlu, Nagam;Zyryanov, Grigory V. [Bioorganic Chemistry,2020,vol. 95]
![1-{4-[(4-hydroxybenzylidene)amino]phenyl}ethanone](/CAS/20180711/GIF/114569-19-6.gif)
114569-19-6
1 suppliers
inquiry

100-10-7
602 suppliers
$5.00/1g
![(2E)-1-(4-aminophenyl)-3-[4-(dimethylamino)phenyl]prop-2-en-1-one](/CAS/20140525/GIF/CB02131373.gif)
126443-09-2
9 suppliers
$126.00/500mg