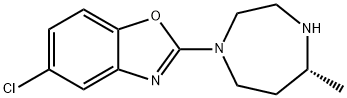
(R)-5-chloro-2-(5-Methyl-1,4-diazepan-1-yl)benzo[d]oxazole synthesis
- Product Name:(R)-5-chloro-2-(5-Methyl-1,4-diazepan-1-yl)benzo[d]oxazole
- CAS Number:1266975-27-2
- Molecular formula:C13H16ClN3O
- Molecular Weight:265.74
![tert-butyl 2-((5-chlorobenzo[d]oxazol-2-yl)(3-oxobutyl)aMino)ethylcarbaMate](/CAS/GIF/1276666-10-4.gif)
1276666-10-4
31 suppliers
inquiry
![(R)-5-chloro-2-(5-Methyl-1,4-diazepan-1-yl)benzo[d]oxazole](/CAS/GIF/1266975-27-2.gif)
1266975-27-2
71 suppliers
$374.00/250mg
Yield: 94.2 % ee
Reaction Conditions:
Stage #1:{2-[(5-chlorobenzoxazol-2-yl)-(3-oxo-butyl)amino]ethyl}carbamic acid tert-butyl ester with hydrogenchloride;water at 20 - 30; for 10 h;
Stage #2: with potassium phosphate in toluene; pH=> 10 at 35;
Stage #3: with formic acid;triethylamine;(CH3C6H4CH(CH3)2)RuCl(NH2CH(C6H5)CH(C6H5)NSO2C6H2(CH(CH3)2)3) in toluene;acetonitrile at -5; for 24 h;Inert atmosphere;
Steps:
6
A pressure flask was charged with Ligand (9) (2.41 g, 5.02mmole), ruthenium cymene dichloride dimer(1.54g, 2.51mmole), triethylamine (l.Olg, 10.04mmole), and acetonitrile (144.5mls). The solution was heated to 80C for 2hr then cooled to ambient and stored 145.0mls of solution in inert environment.A reactor was charged with 2N HCI solution (52.4mls). To this was then chargedMVK adduct (10g, 26,2mmol) in 4 separate additions of 2.5g each. At 20C the reaction was stirred for 4hrs then heated the slurry to 30C for an additional 6hrs and defined conversion. No starting MVK adduct was present following 6hrs at 30C, and began neutralization of the hydrochloride salt by charging lOOmls of toluene to the reactor. Then to this charge, added 50% potassium phosphate tribasic (66, 8g, 157mmol), checked pH to ensure that it was greater than 10 and proceeded to warm batch to 35°C. Phase separated the toluene layer and back extracted the aqueous layer with an additional 25 mis of toluene, then again phase separated and combined the toluene layers. Distilled the toluene to azeotropicaily dry system down to 60mls total of solution.Took 20mls of the above forementioned toluene solution or 8.72mmoles based on MVK adduct charged and to it charged 13,3mls of toluene and 28.3mls of acetonitrile. Cooled the reaction solution down to -5C. To the reaction vessel then charged triethylamine (0.882g, 8.72mmole). Then to this charged previous prepared ruthenium catalyst( 0.1744mmole). To the vessel then charged formic acid (0.40 lg, 8.72mmole) while maintaining temperature at -5C. Once feed of formic acid was complete, started 24hr age of batch. During this age charged formic acid (0.802g, 17.44mmole) and began purging vessel with nitrogen. After 24hrs determined the conversion and ee of reaction by LC. (98.4% conv, 94.2%ee)
References:
MERCK SHARP & DOHME CORP.;MERCK SHARP & DOHME LIMITED;BAXTER, Carl, A.;CLEATOR, Edward;KRSKA, Shane, W.;SHEEN, Faye;STEWART, Gavin;STROTMAN, Neil;WALLACE, Debra, J.;WRIGHT, Timothy WO2012/148553, 2012, A1 Location in patent:Page/Page column 47-48

1276666-13-7
23 suppliers
inquiry
![(R)-5-chloro-2-(5-Methyl-1,4-diazepan-1-yl)benzo[d]oxazole](/CAS/GIF/1266975-27-2.gif)
1266975-27-2
71 suppliers
$374.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![(R)-5-chloro-2-(5-Methyl-1,4-diazepan-1-yl)benzo[d]oxazole](/CAS/GIF/1266975-27-2.gif)
1266975-27-2
71 suppliers
$374.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![(R)-5-chloro-2-(5-Methyl-1,4-diazepan-1-yl)benzo[d]oxazole](/CAS/GIF/1266975-27-2.gif)
1266975-27-2
71 suppliers
$374.00/250mg
![2-Butanone, 4-[(5-chloro-2-benzoxazolyl)[2-[(methylsulfonyl)oxy]ethyl]amino]-](/CAS/20210305/GIF/1383717-11-0.gif)
1383717-11-0
0 suppliers
inquiry
![(R)-5-chloro-2-(5-Methyl-1,4-diazepan-1-yl)benzo[d]oxazole](/CAS/GIF/1266975-27-2.gif)
1266975-27-2
71 suppliers
$374.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry