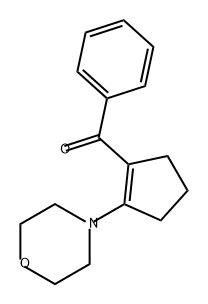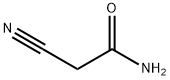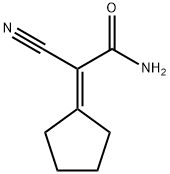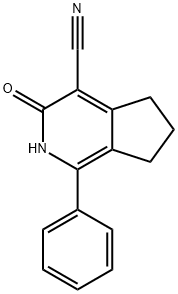
3-Oxo-1-phenyl-3,5,6,7-tetrahydro-2H-[2]pyrindine-4-carbonitrile synthesis
- Product Name:3-Oxo-1-phenyl-3,5,6,7-tetrahydro-2H-[2]pyrindine-4-carbonitrile
- CAS Number:126921-96-8
- Molecular formula:C15H12N2O
- Molecular Weight:236.27
Yield:-
Reaction Conditions:
with diethylamine in benzene; for 10 h;Reflux;
Steps:
1-Alkyl(aryl)-3-oxo-3,5,6,7-tetrahydro-2H-cyclopenta[c]pyridine-4-carbonitriles 2a-2h and 1-alkyl-(aryl)-3-oxo-2,3,5,6,7,8-hexahydroisoquinoline-4-carbonitriles 2i-2p (general procedure).
General procedure: A solution ofcyclopentanone (1a) or cyclohexanone (1b) (0.1 mol),morpholine (8.7 g, 0.1 mol), and a catalytic amount ofp-toluenesulfonic acid in anhydrous benzene (100 mL)was refluxed for 5 h. Triethylamine (10.1 g, 0.1 mol)was added to the reaction mixture, and the correspondingacyl chloride (0.1 mol) was then added dropwise,maintaining the temperature at 35-40°C for 6 h.Cyano acetamide (8.4 g, 0.1 mol) and diethylamine(7.3 g, 0.1 mol) were added, and the mixture was refluxedfor 10 h. After cooling, the crystalline solid wasfiltered off, washed with water, dried, and recrystallizedfrom DMF. The solvent was distilled off from the benzenefiltrate, the dry residue was treated with diethylether, and the crystalline solid precipitated after a timewas filtered off and recrystallized from DMF.
References:
Geronikaki, A.;Hakobyan, E. K.;Hovakimyan, A. A.;Kartsev, V. G.;Sirakanyan, S. N. [Russian Journal of Organic Chemistry,2021,vol. 57,# 10,p. 1748 - 1752][Zh. Org. Khim.,2021,vol. 57,p. 1748 - 1752]

120-92-3
416 suppliers
$11.00/25g
![3-Oxo-1-phenyl-3,5,6,7-tetrahydro-2H-[2]pyrindine-4-carbonitrile](/CAS/20180808/GIF/126921-96-8.gif)
126921-96-8
4 suppliers
inquiry
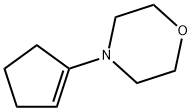
936-52-7
113 suppliers
$38.00/25g
![3-Oxo-1-phenyl-3,5,6,7-tetrahydro-2H-[2]pyrindine-4-carbonitrile](/CAS/20180808/GIF/126921-96-8.gif)
126921-96-8
4 suppliers
inquiry
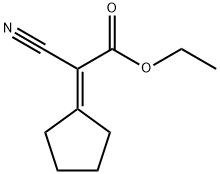
5407-83-0
19 suppliers
$28.60/250mg

100-52-7
953 suppliers
$20.37/250g
![3-Oxo-1-phenyl-3,5,6,7-tetrahydro-2H-[2]pyrindine-4-carbonitrile](/CAS/20180808/GIF/126921-96-8.gif)
126921-96-8
4 suppliers
inquiry