
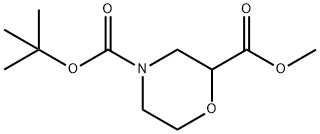
4-tert-butyl 2-ethyl 2-methylmorpholine-2,4-dicarboxylate synthesis
- Product Name:4-tert-butyl 2-ethyl 2-methylmorpholine-2,4-dicarboxylate
- CAS Number:1269755-24-9
- Molecular formula:C12H21NO5
- Molecular Weight:259.3
500789-41-3
63 suppliers
$21.00/100mg

74-88-4
345 suppliers
$15.00/10g

1269755-24-9
18 suppliers
inquiry
Yield:1269755-24-9 22%
Reaction Conditions:
Stage #1: 4-(t-butyloxycarbonyl)morpholine-2-carboxylic acid methyl esterwith n-butyllithium;diisopropylamine in tetrahydrofuran at -78; for 1 h;
Stage #2: methyl iodide in tetrahydrofuran at 20; for 16 h;
Stage #3: with lithium diisopropyl amide in tetrahydrofuran at -78 - 20;
Steps:
4-tert-Butyl 2-methyl 2-methylmorpholine-2,4-dicarboxylate
Diisopropylamine (0.35 mL, 2.48 mmol) was added dropwise to 2.5Mbutyllithium solution (1 mL) in THF (2 mL) at -74°C. The mixture was allowed to warm to r.t. whilst stirring for 1 h. The mixture was re-cooled and Intermediate 136 (478 mg, 1.95 mmol) in THF (5 mL) was added dropwise at -78°C. The reaction mixture was allowed to stir for 1 h, then iodomethane (0.15 mL, 2.41 mmol) was added dropwise. The reaction mixture was allowed to warm to room temperature and was stirred for 16 h. Thereaction mixture was cooled to -78°C and 2M lithium dipropan-2-ylazanide (10 mL) was added dropwise. The reaction mixture was allowed to stir for 1 h. lodomethane (0.05 mL, 0.8 mmol) was added dropwise at -78°C. The reaction mixture was allowed to stir at r.t. overnight. The reaction mixture was cooled to 0°C and quenched with saturated aqueous ammonium chloride solution (10 mL), diluted with water (20 mL) and extractedwith ethyl acetate (3 x 20 mL). The combined organic layers were washed with brine (25 mL), dried over sodium sulfate and concentrated under vacuum. The crude residue was purified onto a 25g silica cartridge, eluting with a gradient of 0-25% ethyl acetate in heptane, to afford the title compound (110 mg, 22%) as a pale yellow oil. oH (500 MHz, CD3OD) 4.39 (d, J 13.2 Hz, 1H), 3.79-3.70 (m, 6H), 2.99 (s, 1H), 2.85 (d, J 12.3 Hz, 1H),1.46 (s, 9H), 1.33 (s, 3H).
References:
WO2014/9295,2014,A1 Location in patent:Page/Page column 176

500789-41-3
63 suppliers
$21.00/100mg

1269755-24-9
18 suppliers
inquiry
189321-66-2
143 suppliers
$5.00/250mg

1269755-24-9
18 suppliers
inquiry