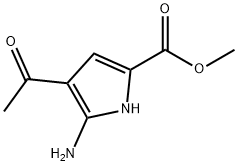
1269824-46-5 synthesis
- Product Name:1269824-46-5
- CAS Number:1269824-46-5
- Molecular formula:C8H10N2O3
- Molecular Weight:182.18

1269822-94-7
7 suppliers
inquiry

1269824-46-5
8 suppliers
inquiry
Yield: 87.5%
Reaction Conditions:
with iron;acetic acid at 45 - 100; for 0.5 h;
Steps:
A solution of Methyl 4-acetyl-5-amino-lH-pyrrole-2-carboxylate 31d (10 g, 47.00 mmol) in acetic acid (60 mL) was heated at 45 °C. To a homogenous solution iron powder (7.87 g, 55.85 mmol) was added and continued stirring at 45 °C. After 30 min the reaction temperature reaches 100 °C, and the reaction mixture becomes heterogeneous. The solid obtained was dissolved in 10% aq. ammonia in methanol (100 mL), filtered through celite and concentrated in vacuum. The residue obtained was purified by flash chromatography (silica gel, eluting with CMA 80 in chloroform 0 to 50%) to afford methyl 4-acetyl-5-amino-lH-pyrrole-2-carboxylate 31e (7.5 g. 87.5%) as a light brown solid. 1HNMR (300 MHz, DMSO) δ 10.87 (s, 1H, D20 exchangeable), 7.03 (d, J= 2.4, 1H), 6.35 (s, 2H, D20 exchangeable), 3.71 (s, 3H), 2.21 (s, 3H). MS (ES+1) 183.2 (M+l)
References:
BIOCRYST PHARMACEUTICALS, INC.;BABU, Yarlagadda S.;KOTIAN, Pravin L.;KUMAR, V. Satish;WU, Minwan;LIN, Tsu-Hsing WO2011/31554, 2011, A2 Location in patent:Page/Page column 143
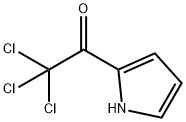
35302-72-8
178 suppliers
$10.00/5g

1269824-46-5
8 suppliers
inquiry
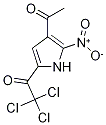
1269822-93-6
6 suppliers
inquiry

1269824-46-5
8 suppliers
inquiry
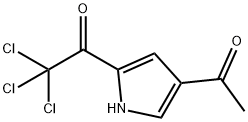
72652-34-7
22 suppliers
$362.00/500mg

1269824-46-5
8 suppliers
inquiry