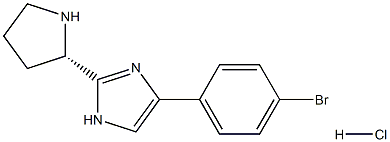
(S)-4-(4-bromophenyl)-2-(pyrrolidin-2-yl)-1H-imidazole hydrochloride synthesis
- Product Name:(S)-4-(4-bromophenyl)-2-(pyrrolidin-2-yl)-1H-imidazole hydrochloride
- CAS Number:1272654-85-9
- Molecular formula:C13H15BrClN3
- Molecular Weight:328.6353
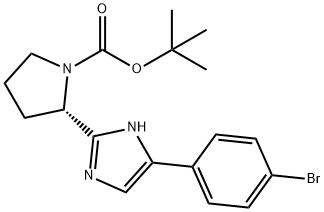
1007882-04-3
59 suppliers
inquiry

1272654-85-9
6 suppliers
inquiry
Yield:1272654-85-9 98%
Reaction Conditions:
with hydrogenchloride in 1,4-dioxane;methanol at 20; for 4 h;
Steps:
A mixture of (S)-tert-butyl 2-(5-(4-bromophenyl)-lH-imidazol-2-yl)pyrrolidine-l -carboxylate (2.45 g, 6.25 mmol,) and 4.0 M HCl/dioxane solution (15 mL) in methanol (30 mL) was stirred at room temperature for 4h. Concentrate the reaction in vacuo. The crude mixture was made basic with saturated sodium bicarbonate solution and was extracted from the aqueous layer with ethyl acetate. The combined organic fractions were washed with a saturated sodium chloride solution and dried over magnesium sulfate, filtered and concentrated to afford, (S)-5-(4- bromophenyl)-2-(pyrrolidin-2-yl)-lH-imidazole hydrochloride as an orange solid, (1.80 g, 98%): ESI-LRMS m/e calcd for Ci3Hi4BrN3 HC1 [M+] 328.5, found 293 [M+H+] (free base).
References:
WO2013/53657,2013,A1 Location in patent:Page/Page column 41; 42
1007881-98-2
37 suppliers
inquiry

1272654-85-9
6 suppliers
inquiry
15761-39-4
602 suppliers
$5.00/10g

1272654-85-9
6 suppliers
inquiry
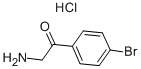
5467-72-1
190 suppliers
$12.90/1g

1272654-85-9
6 suppliers
inquiry
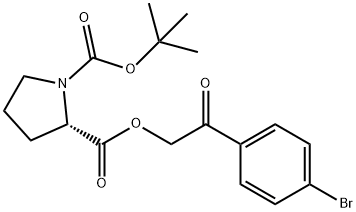
128120-93-4
1 suppliers
inquiry

1272654-85-9
6 suppliers
inquiry