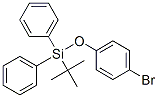
Silane, (4-bromophenoxy)(1,1-dimethylethyl)diphenyl- synthesis
- Product Name:Silane, (4-bromophenoxy)(1,1-dimethylethyl)diphenyl-
- CAS Number:127481-94-1
- Molecular formula:
- Molecular Weight:0
106-41-2
495 suppliers
$13.00/5g

58479-61-1
407 suppliers
$10.00/25g

127481-94-1
3 suppliers
inquiry
Yield:127481-94-1 100%
Reaction Conditions:
Stage #1: 4-bromo-phenolwith 1H-imidazole in N,N-dimethyl-formamide at 20; for 0.166667 h;Inert atmosphere;
Stage #2: tert-butylchlorodiphenylsilane in N,N-dimethyl-formamide at 20; for 19 h;Inert atmosphere;
Steps:
4-Bromophenyl tert-Butyldiphenylsilyl Ether (7)
Imidazole (2.37 g, 34.8 mmol) was added to a solution of 4-bromophenol (3.05 g, 17.6 mmol) in DMF (36 mL) and the mixture was stirred for 10 min at r.t., before t-BuPh2SiCl (7.0 mL, 7.4 g, 27 mmol) was added dropwise. The mixture was stirred for a further 19 h at r.t. and then aq 1 M HCl (40 mL) was added. The aqueous layer was separated and extracted with CH2Cl2. The combined organic layers were washed with sat. aq NH4Cl, then with brine, and dried over MgSO4. Removal of the solvent in vacuo and column chromatography (silica gel, isohexane/EtOAc, 15:1 to 12:1) of the residue provided compound 7 as a colorless oil; yield: 7.24 g (100%). 1H NMR (500 MHz, CDCl3): δ = 1.09 (s, 9 H), 6.61-6.64 (m, 2 H), 7.15-7.18 (m, 2 H), 7.35-7.38 (m, 4 H), 7.41-7.45 (m, 2 H), 7.68-7.69 (m, 4 H). 13C NMR and DEPT (125 MHz, CDCl3): δ = 19.40 (C), 26.42 (3 CH3),113.32 (C), 121.46 (2 CH), 127.84 (4 CH), 130.03 (2 CH), 132.06 (2 CH), 132.40 (2 C), 135.44 (4 CH), 154.72 (C). MS (EI): m/z (%) = 412 (9), 410 (9, [M+]), 355 (100), 353 (100), 273 (76), 197 (29), 152 (23), 57 (25). For further spectroscopic data, see ref. 7.
References:
L?sle, Valerie;Kataeva, Olga;Kn?lker, Hans-Joachim [Synthesis,2021,vol. 53,# 2,p. 359 - 364]