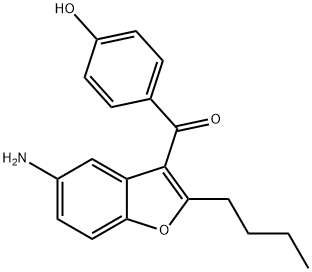
(5-aMino-2-butyl-1-benzofuran-3-yl)(4-hydroxyphenyl)Methanone synthesis
- Product Name:(5-aMino-2-butyl-1-benzofuran-3-yl)(4-hydroxyphenyl)Methanone
- CAS Number:1278585-68-4
- Molecular formula:C19H19NO3
- Molecular Weight:309.36
141645-16-1
240 suppliers
$10.00/5g

1278585-68-4
16 suppliers
inquiry
Yield:1278585-68-4 78.3%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in ethanol at 50; under 3750.38 Torr; for 6 h;
Steps:
12 (5 -amino-2-butyl- 1 -benzofuran-3 -yl)(4-hydroxyphenyl)methanone (XI)
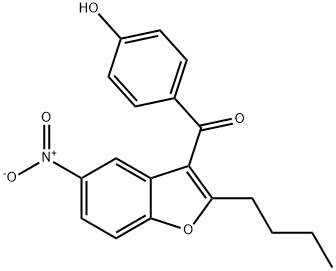
33.9 g of (2-butyl-5-nitro-l-benzofuran-3-yl)(4-hydroxyphenyl)methanone (X) was placed in a reactor and 150 ml of abs ethanol was added. After stirring at 20-25°C for 5 minutes 1.5 g of wet Pd/C catalyst of 10 w/w% was added. The reactor was closed and under stirring was flushed 3 times with nitrogen, 3 times with hydrogen and 3 times with nitrogen. The reactor was set under hydrogen pressure of 5 bar and the temperature was raised to 50°C. Hydrogenation was carried out for 6 hours then the reaction mixture was cooled to room temperature. At a pressure of 1 bar 75 ml of acetone was added and the mixture was stirred until complete dissolution. The catalyst was filtered out, the solvent was evaporated. Mass of residual material: 29.8 g The crude material was dissolved in 300 ml of abs ethanol and heated to 80°C. It was cooled to 10°C and the separated crystals filtered. Yield of the pure compound: 23.36 g (78.3%). Purity (HPLC: 100%). Mp.: 209.1-210.0°C 1H NMR : 10.4(s, 1H); 7.70(d, J=8.7 Hz, 2H); 7.28(d, J=8.7Hz ,1H); 6.9 l(d, J=8.7 Hz, 2H); 6.60(dd, J=8.7, 2.5Hz, 1H) ; 6.54(d, J=2.5 Hz, 1H); 4.92(s, 2H); 2.76(t, J=7.5 Hz, 2H); 1.65 (5', J=7.5 Hz, 2H); 1.26(6', J=7.5Hz, 2H); 0.84(t, J=7.5Hz, 3H).
References:
WO2013/14480,2013,A1 Location in patent:Page/Page column 24