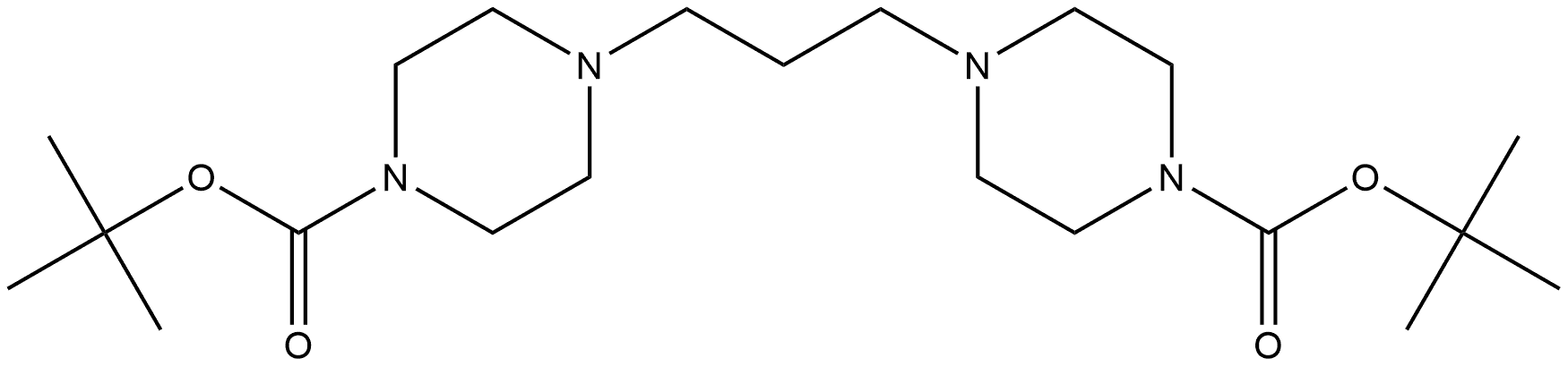
1-Piperazinecarboxylic acid, 4,4'-(1,3-propanediyl)bis-, 1,1'-bis(1,1-dimethylethyl) ester synthesis
- Product Name:1-Piperazinecarboxylic acid, 4,4'-(1,3-propanediyl)bis-, 1,1'-bis(1,1-dimethylethyl) ester
- CAS Number:1279815-19-8
- Molecular formula:C21H40N4O4
- Molecular Weight:412.57
Yield:1279815-19-8 78%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 20; for 24 h;
Steps:
Di-tert-butyl 4,4'-(propane-1,3-diyl)bis(piperazine-1-carboxylate) 10
To a stirred solution of 300 mg (1.61 mmol) of mono Boc-piperazine 6 in 5 mL of dry DMF were added 82.0 μL (163 mg, 0.80 mmol) of 1,3-dibromopropane 8 and 890 mg (6.44 mmol) of K2CO3 at room temperature.
After 24 h, reaction mixture was diluted with 25 mL of water and extracted with two 50 mL portions of ethyl acetate.
The organic phase was dried (MgSO4) and concentrated under diminished pressure.
The residue was purified by chromatography on a silica gel column (10*2 cm).
Elution with 19:1 ethyl acetate/methanol gave desired product 10 as a colorless solid: yield 521 g (78%); silica gel TLC Rf 0.27 (9:1 ethyl acetate/methanol); 1H NMR (CDCl3) δ 1.46 (s, 18H), 1.64-1.74 (m, 2H), 2.35-2.39 (m, 12H) and 3.41-3.44 (m, 8H); 13C NMR (CDCl3) δ 28.4, 31.4, 36.4, 53.1, 56.6, 79.5 and 154.7.
References:
US2019/106396,2019,A1 Location in patent:Paragraph 0040; 0041; 0047

