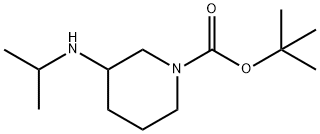
tert-butyl 3-(isopropylamino)piperidine-1-carboxylate synthesis
- Product Name:tert-butyl 3-(isopropylamino)piperidine-1-carboxylate
- CAS Number:1282742-29-3
- Molecular formula:C13H26N2O2
- Molecular Weight:242.36
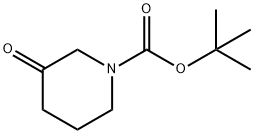
98977-36-7
428 suppliers
$6.00/1g
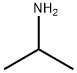
75-31-0
413 suppliers
$14.00/25mL

1282742-29-3
15 suppliers
$495.55/5MG
Yield:1282742-29-3 82%
Reaction Conditions:
Stage #1: 3-oxo-piperidine-1-carboxylic acid tert-butyl ester;isopropylaminewith methanol at 20; for 1 h;
Stage #2: with sodium tetrahydroborate at 0 - 20;
Stage #3: with waterCooling with ice;
Steps:
52
Synthesis is ieri-butyl 3-(isopropylamino)piperidine-l-carboxylate: A solution teri-butyl 3-oxopiperidine-l-carboxylate (2 g, 10.03 mmol) and 2-aminopropane (710 mg, 12. mmol) in MeOH (40 mL) was stirred at rt for 1 h. To the solution was added NaBH4 (758 n 20.06 mmol) at 0 °C and the reaction mixture was stirred at rt for 12h. The reaction mixture w diluted with ice-cooled water and the solvent was reduced to afford a residue which was w dissolved in CH2C12 and washed with water. The organic layer was dried over Na2S04, concentrated in vacuo and purified by column chromatography (silica gel, gradient MeOH in CH2C12) to afford the titled compound (2 g, 82%). 1H NMR (400 MHz, CDC13): δ 4.01-3.90 (m, IH), 3.78 (dt, J = 3.6, 9.2 Hz, IH), 3.48 (s, IH), 2.96 (heptate, J = 6.4 Hz, IH), 2.92-2.79 (m, IH), 2.69-2.55 (m, IH), 1.90-1.87 (m, IH), 1.68-1.61 (m, IH), 1.46 (s, 9H), 1.29-1.18 (m, 2H), 1.05 (d, J = 6.0 Hz, 6H). ES-MS: m/z [M+l] = 243.
References:
WO2012/58645,2012,A1 Location in patent:Page/Page column 115-116