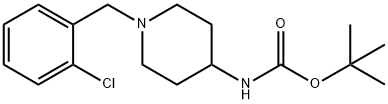
tert-Butyl 1-(2-chlorobenzyl)piperidin-4-ylcarbamate synthesis
- Product Name:tert-Butyl 1-(2-chlorobenzyl)piperidin-4-ylcarbamate
- CAS Number:1286273-10-6
- Molecular formula:C17H25ClN2O2
- Molecular Weight:324.85
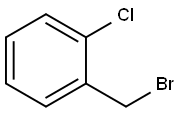
611-17-6
193 suppliers
$9.00/1g
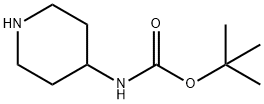
73874-95-0
449 suppliers
$10.00/250mg

1286273-10-6
7 suppliers
$309.00/1g
Yield:1286273-10-6 97%
Reaction Conditions:
with potassium carbonate in dichloromethane at 20;
Steps:
2 Synthesis of Compound 2-b
Compound 1 (500mg, 2.5mmol) was dissolved in DCM (50ml) and then 2-chlorobenzyl bromide (0.37ml, 2.74mmol) was added thereto.K2CO3 (517 mg, 3.74 mmol) was added and stirred at room temperature. After the reaction was completed, water was added and extracted twice with DCM. ObtainedThe organic layer was dried over anhydrous MgSO 4 and filtered. The filtrate was concentrated under reduced pressure, and then silica column chromatography.Purification with (Hex: EA = 1: 1) gave the compound 2-b as a white solid (791 mg, 97%).
References:
KR101548927,2015,B1 Location in patent:Paragraph 0069-0072

89-98-5
634 suppliers
$15.00/25g

73874-95-0
449 suppliers
$10.00/250mg

1286273-10-6
7 suppliers
$309.00/1g