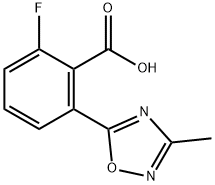
2-Fluoro-6-(3-methyl-1,2,4-oxadiazol-5-yl)benzoic acid synthesis
- Product Name:2-Fluoro-6-(3-methyl-1,2,4-oxadiazol-5-yl)benzoic acid
- CAS Number:1293285-27-4
- Molecular formula:C10H7FN2O3
- Molecular Weight:222.17
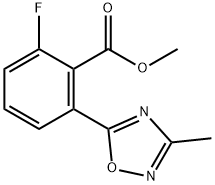
1293285-23-0
1 suppliers
inquiry

1293285-25-2
1 suppliers
inquiry

1293285-27-4
4 suppliers
inquiry

1293285-18-3
2 suppliers
inquiry
Yield:1293285-18-3 15%
Reaction Conditions:
in water;Conversion of starting material;
Steps:
15.C
Step C: 3-Fluoro-2-(3-methyl-1 ,2,4-oxadiazol-5-yl)benzoic acid. To the mixture of products from Step B (477 mg, 1 .88 mmol) in t-BuOH (9 ml_) was added NaOAc (156 mg, 1 .88 mmol). The mixture was heated at 90 C for 50 h and then concentrated in vacuo. This resulted in four products. The residue was dissolved in 1 M K2CO3 and extracted with DCM to isolate methyl 2-fluoro- 6-(3-methyl-1 ,2,4-oxadiazol-5-yl)benzoate and methyl 3-fluoro-2-(3-methyl- 1 ,2,4-oxadiazol-5-yl)benzoate along with unreacted (Z)-methyl 2-((((1 - aminoethylidene)amino)oxy)carbonyl)-3-fluorobenzoate. The aqueous layer was then acidified with concentrate HCI and extracted with DCM. The combined organic layers from this extraction were dried over Na2SO4, filtered and concentrated in vacuo. The acid isomers were purified on a Prep Agilent system with a XBridge Cis OBD 50x100 mm column eluting with 5 to 99% 0.05% NH4OH in H2O/ACN over 17 min to afford the desired product (63 mg, 15%) as a white solid after acidification with 1 M HCI in Et2O. MS (ESI) mass calculated for Ci0H7FN2O3, 222.04; m/z found, 223.0.
References:
WO2011/50202,2011,A1 Location in patent:Page/Page column 58-59

67-56-1
738 suppliers
$9.00/25ml
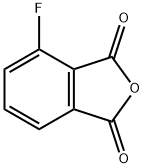
652-39-1
256 suppliers
$9.00/1g
1141922-26-0
1 suppliers
inquiry

1293285-23-0
1 suppliers
inquiry

1293285-25-2
1 suppliers
inquiry

1293285-27-4
4 suppliers
inquiry

1293285-18-3
2 suppliers
inquiry
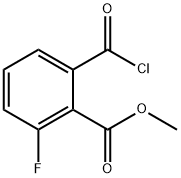
1256593-41-5
1 suppliers
inquiry

1293285-27-4
4 suppliers
inquiry