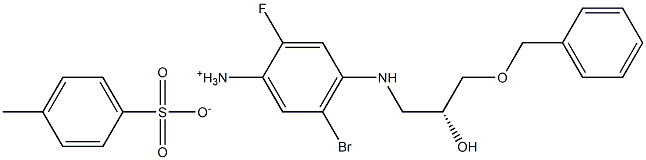
(R)-4-((3-(benzyloxy)-2-hydroxypropyl)amino)-5-bromo-2-fluorobenzenaminium4-methylbenzenesulfonate synthesis
- Product Name:(R)-4-((3-(benzyloxy)-2-hydroxypropyl)amino)-5-bromo-2-fluorobenzenaminium4-methylbenzenesulfonate
- CAS Number:1294504-64-5
- Molecular formula:C23H26BrFN2O5S
- Molecular Weight:541.4304
![2-Propanol, 1-[(2-broMo-5-fluoro-4-nitrophenyl)aMino]-3-(phenylMethoxy)-, (2R)-](/CAS/20150408/GIF/1342896-73-4.gif)
1342896-73-4
6 suppliers
inquiry
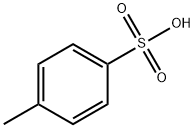
104-15-4
722 suppliers
$133.00/500g

1294504-64-5
17 suppliers
inquiry
Yield:97 % ee
Reaction Conditions:
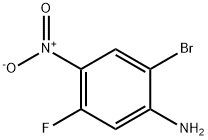
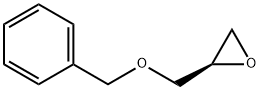
Stage #1: (R)-1-(benzyloxy)-3-(2-bromo-5-fluoro-4-nitrophenylamino)propan-2-olwith platinum on carbon;hydrogen in Isopropyl acetate at 30; under 750.075 Torr;
Stage #2: toluene-4-sulfonic acid in dichloromethane;
Steps:
1
The hydrogenator was charged with 5 wt % Pt(S)/C (1.5 mol %) and the mixture was stirred under N2 at 30° C. (internal temperature). The reaction was flushed with N2 followed by hydrogen. The hydrogenator pressure was adjusted to 1 bar of hydrogen and the mixture was stirred rapidly (>1200 rpm). At the end of the reaction, the catalyst was filtered through a pad of Celite and washed with dichloromethane (10 vol). The filtrate was concentrated in vacuo. Any remaining isopropyl acetate was chased with dichloromethane (2 vol) and concentrated on a rotavap to dryness. (0619) The resulting residue was dissolved in dichloromethane (10 vol). p-Toluenesulfonic acid monohydrate (1.2 equiv) was added and stirred overnight. The product was filtered and washed with dichloromethane (2 vol) and suction dried. The wetcake was transferred to drying trays and into a vacuum oven and dried at 45° C. with N2 bleed until constant weight was achieved. p-Toluenesulfonic acid salt of (R)-1-((4-amino-2-bromo-5-fluorophenyl)amino)-3-(benzyloxy)propan-2-ol was isolated as an off-white solid. (0620) Chiral purity was determined to be >97% ee.
References:
US10980746,2021,B2 Location in patent:Page/Page column 105; 106
952664-69-6
116 suppliers
$7.00/250mg

104-15-4
722 suppliers
$133.00/500g
14618-80-5
274 suppliers
$5.00/1g

1294504-64-5
17 suppliers
inquiry
![2-Propanol, 1-[(4-aMino-2-broMo-5-fluorophenyl)aMino]-3-(phenylMethoxy)-, (2R)-](/CAS/20150408/GIF/1294504-63-4.gif)
1294504-63-4
3 suppliers
inquiry

104-15-4
722 suppliers
$133.00/500g

1294504-64-5
17 suppliers
inquiry
2369-13-3
246 suppliers
$19.00/5g

1294504-64-5
17 suppliers
inquiry

952664-69-6
116 suppliers
$7.00/250mg

1294504-64-5
17 suppliers
inquiry