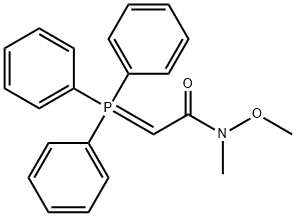
N-METHOXY-N-METHYL(TRIPHENYL-PHOSPHORANYLIDENE)ACETAMIDE synthesis
- Product Name:N-METHOXY-N-METHYL(TRIPHENYL-PHOSPHORANYLIDENE)ACETAMIDE
- CAS Number:129986-67-0
- Molecular formula:C22H22NO2P
- Molecular Weight:363.39
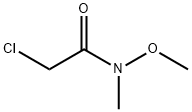
67442-07-3
200 suppliers
$6.00/5g
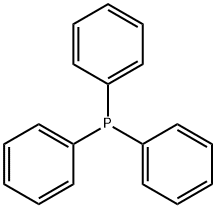
603-35-0
700 suppliers
$5.00/5 g

129986-67-0
44 suppliers
$55.00/100mg
Yield:129986-67-0 98%
Reaction Conditions:
in acetonitrile at 80; for 20 h;
Steps:
177.1 Step 1: N-Methoxy-N-methyl-2-(triphenyl-15-phosphanylidene)acetamide
Step 1:
N-Methoxy-N-methyl-2-(triphenyl-15-phosphanylidene)acetamide
A mixture of (2-chloro-N-methoxy-N-methylacetamide (13.7 g, 0.1 mol) and triphenylphosphane (26.2 g, 0.1 mol) in acetonitrile (200 mL) was heated to 80° C. and held for 20 h.
The mixture was cooled and concentrated to remove the solvent below 40° C.
The residue was dissolved in dichloromethane (200 mL), followed by 2 N KOH (100 mL).
The resulting mixture was stirred at 20° C. for 1 h.
Phase separation, the organic layer was washed with brine (200 mL*3), dried over Na2SO4 and filtered.
The filtrate was concentrated in vacuum to afford N-methoxy-N-methyl-2-(triphenyl-15-phosphanylidene) acetamide (36 g, 0.1 mol, 98%) as a yellow solid. ESI-MS (EI+, m/z): 364.4 [M+H]+.
References:
Navitor Pharmaceuticals, Inc.;Fetalvero, Kristina Michelle;Narayan, Sridhar;O'Neill, David John;Saiah, Eddine;Sengupta, Shomit US2017/114080, 2017, A1 Location in patent:Paragraph 0505
134833-83-3
35 suppliers
$2477.48/5G

603-35-0
700 suppliers
$5.00/5 g

129986-67-0
44 suppliers
$55.00/100mg

603-35-0
700 suppliers
$5.00/5 g

129986-67-0
44 suppliers
$55.00/100mg