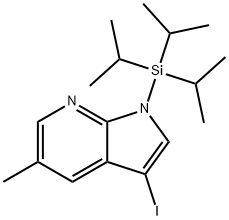
1H-Pyrrolo[2,3-b]pyridine, 3-iodo-5-methyl-1-[tris(1-methylethyl)silyl]- synthesis
- Product Name:1H-Pyrrolo[2,3-b]pyridine, 3-iodo-5-methyl-1-[tris(1-methylethyl)silyl]-
- CAS Number:1303426-80-3
- Molecular formula:C17H27IN2Si
- Molecular Weight:414.4
![1H-Pyrrolo[2,3-b]pyridine, 5-methyl-1-[tris(1-methylethyl)silyl]-](/CAS/GIF/918523-66-7.gif)
918523-66-7
2 suppliers
inquiry
![1H-Pyrrolo[2,3-b]pyridine, 3-iodo-5-methyl-1-[tris(1-methylethyl)silyl]-](/CAS/20210111/GIF/1303426-80-3.gif)
1303426-80-3
0 suppliers
inquiry
Yield: 75%
Reaction Conditions:
with N-iodo-succinimide in dichloromethane at 20;
Steps:
3.1
Example 3; Synthesis of 3-iodo-5-methyl-1-triisopropylsilanyl-1H-pyrrolo[2,3-b]pyridine 83-Iodo-5-methyl-1-triisopropylsilanyl-1H-pyrrolo[2,3-b]pyridine 8 is prepared in one step from 5-methyl-1-triisopropylsilanyl-1H-pyrrolo[2,3-b]pyridine 6 as shown in Scheme 3. Step 1-Preparation of 3-iodo-5-methyl-1-triisopropylsilanyl-1H-pyrrolo[2,3-b]pyridine (8)5-Methyl-1-triisopropylsilanyl-1H-pyrrolo[2,3-b]pyridine (6, 1.1 g, 3.8 mmol) and 10 mL of dichloromethane are combined in a round bottom flask and stirred for 10 minutes. A slurry of N-iodosuccinimide (1.0 g, 4.6 mmol) in 5 mL of dichloromethane is added and stirred at room temperature overnight. The reaction is quenched with sodium thiosulfate (20 mL, 1M in water) and the aqueous layer is extracted with ethyl acetate. The combined organic layer is washed with water and brine, dried with sodium sulfate, filtered and the filtrate concentrated under vacuum. The resulting material is purified by silica gel column chromatography, eluting with ethyl acetate and hexanes. Appropriate fractions are combined and concentrated under vacuum to provide the desired compound as a light yellow oil (8, 1.2 g, 75%). MS (ESI) [M+H+]+=415.08.
References:
Zhang, Jiazhong;Ibrahim, Prabha N.;Spevak, Wayne;Tsai, James;Ewing, Todd;Zhang, Ying;Zhang, Chao US2011/263595, 2011, A1 Location in patent:Page/Page column 79