
1303512-02-8 synthesis
- Product Name:1303512-02-8
- CAS Number:1303512-02-8
- Molecular formula:C22H28ClN3O4
- Molecular Weight:433.93

50-00-0
845 suppliers
$10.00/25g
![2(1H)-Pyridinone, 1-[(4-chlorophenyl)methyl]-3-hydroxy-](/CAS/20210305/GIF/1303512-01-7.gif)
1303512-01-7
1 suppliers
inquiry

57260-71-6
741 suppliers
$5.00/5g

1303512-02-8
16 suppliers
inquiry
Yield: 83.9%
Reaction Conditions:
with acetic acid in ethanol;water at 50; for 19 h;Inert atmosphere;
Steps:
1 Preparation of tert-Butyl 4-((1-(4-chlorobenzyl)-3-hydroxy-2-oxo-1,2-dihydropyridin-4-yl)methyl)piperazine-1-carboxylate (3)
Preparation of tert-butyl 4-((1-(4-chlorobenzyl)-3-hydroxy-2-oxo-1,2-dihydropyridin-4-yl)methyl)piperazine-1-carboxylate (3): to a nitrogen purged 2-L round bottom flask equipped with a mechanical stirrer, upright condenser, and thermometer was charged 1-(4-chlorobenzyl)-3-hydroxypyridin-2(1H)-one (1) (50.0 g, 0.21 mol, 1 equiv.), tert-butyl piperazine-1-carboxylate, (2) (79.0 g, 0.42 mol, 2 equiv.) [CAS No. 57260-71-6] and ethanol (750 mL, 15 parts v/w). The solution was stirred and 37% aqueous formaldehyde (34.7 mL, 0.47 mol, 2.2 equiv.) and acetic acid (36.4 mL, 0.64 mol, 3 equiv.) were added and the solution stirred for 1 hour after which the reaction solution was heated to 50° C. for 18 hours. The reaction mixture was then cooled below room temperature and filtered under vacuum. The resulting solid was rinsed with ethanol (250 mL) and dried under a stream of nitrogen to afford 77.2 g (83.9%) of the desired product. 1H NMR (DMSO-d6) δ ppm 7.4 (d, 2H), 7.3 (d, 2H), 7.2 (d, 1H), 6.2 (d, 1H), 5.1 (s, 2H), 3.4-3.2 (m partly under brs water peak, 6H), 2.3 (m, 4H), 1.4 (s, 9H).13C NMR (DMSO-d6) [observed] δ ppm 157.24, 153.77, 144.36, 136.33, 132.15, 129.67, 128.48, 126.86, 124.56, 107.01, 78.74, 54.68, 52.49, 50.64, 43.48, and 28.03.
References:
Aerpio Therapeutics Inc.;Alberico, Dino US2015/232425, 2015, A1 Location in patent:Paragraph 0212
![2(1H)-Pyridinone, 3-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-](/CAS/20210305/GIF/141667-58-5.gif)
141667-58-5
0 suppliers
inquiry

1303512-02-8
16 suppliers
inquiry
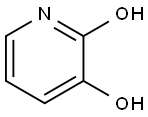
84719-32-4
63 suppliers
inquiry

1303512-02-8
16 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1303512-02-8
16 suppliers
inquiry