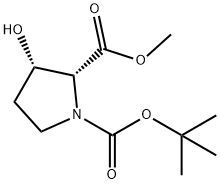
1-tert-butyl 2-methyl (2R,3S)-3-hydroxypyrrolidine-1,2-dicarboxylate synthesis
- Product Name:1-tert-butyl 2-methyl (2R,3S)-3-hydroxypyrrolidine-1,2-dicarboxylate
- CAS Number:130966-41-5
- Molecular formula:C11H19NO5
- Molecular Weight:245.27

130948-04-8
4 suppliers
$918.99/5gm:

130966-41-5
21 suppliers
inquiry
Yield:> 99 % ee
Reaction Conditions:
with Bakers yeast
Steps:
6 Synthesis of the Pro3 Monomer Class; Example 6
The starting material for 37:pro3(2R3S) and 38:pro3(2R3R), the other two stereoisomers in the pro3 class can be synthesized using an established chemoenzymatic route. Non-fermenting bakers yeast reduction of the known ketoester 48 [37] has been used to synthesize the protected cis-3-Hydroxy-(D)-proline 39 with greater than 99% ee (Scheme 8). Gellman and co-workers have used a similar bakers yeast reduction to synthesize the starting material for the synthesis of their Fmoc-AP(Boc) beta-amino acid [38]. The building blocks 37:pro3(2R3S) and 38:pro3(2R3R) can be synthesized using the same approach as was used for 35:pro3(2S3R) and 36:pro3 (2S3S) but starting from cis-3-Hydroxy-(D)-proline.
References:
US2004/77879,2004,A1 Location in patent:Page 15

67-56-1
775 suppliers
$9.00/25ml
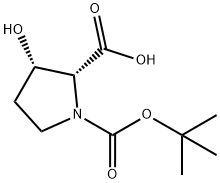
118492-87-8
41 suppliers
$45.00/2.5mg

130966-41-5
21 suppliers
inquiry
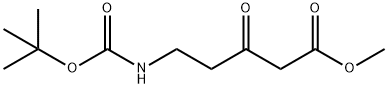
141734-32-9
15 suppliers
inquiry

130966-41-5
21 suppliers
inquiry
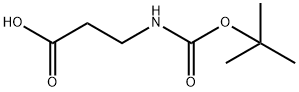
3303-84-2
377 suppliers
$6.00/5g

130966-41-5
21 suppliers
inquiry