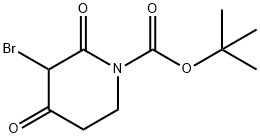
3-BroMo-2,4-dioxo-piperidine-1-carboxylic acid tert-butyl ester synthesis
- Product Name:3-BroMo-2,4-dioxo-piperidine-1-carboxylic acid tert-butyl ester
- CAS Number:1312412-87-5
- Molecular formula:C10H14BrNO4
- Molecular Weight:292.13
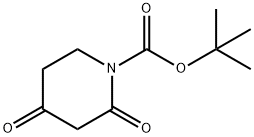
845267-78-9
170 suppliers
$10.00/1g

1312412-87-5
29 suppliers
$80.00/100mg
Yield:1312412-87-5 99%
Reaction Conditions:
with N-Bromosuccinimide in tetrachloromethane at 10 - 15; for 2 h;
Steps:
25.1 Step 1: tert-butyl 3-bromo-2,4-dioxopiperidine-1-carboxylate
To a stirred solution of tert-butyl 2,4-dioxopiperidine-1-carboxylate (1 g, 4.69 mmol) in dry CCl4 (10 mL), N-bromosuccinimide (0.83 g, 4.69 mmol) was added at 10 °C. The reaction mixture was stirred at 10-15 °C for 2 h. It was then evaporated under reduced pressure. Water (10 mL) was added and the desired product was extracted with EtOAc (2 x 30 mL). The combined organic layer was dried over Na2SO4 and concentrated. The resulting crude product was purified by flash column chromatography, affording the title product. Yield: 99% (1.4 g, off white solid). 1H NMR (400 MHz, DMSO-d6): δ 5.50 (s, 1H), 3.74-3.71 (m, 2H), 2.69-2.66 (m, 2H), 1.46 (s, 9H). LCMS: (Method A) 193.8 (M-Boc+H), Rt. 2.93min, 81 .51 % (Max).
References:
WO2016/30443,2016,A1 Location in patent:Page/Page column 96
3303-84-2
373 suppliers
$6.00/5g

1312412-87-5
29 suppliers
$80.00/100mg

24424-99-5
836 suppliers
$13.50/25G

1312412-87-5
29 suppliers
$80.00/100mg