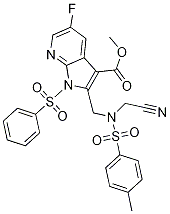
1H-Pyrrolo[2,3-b]pyridine-3-carboxylic acid, 2-[[(cyanoMethyl)[(4-Methylphenyl)sulfonyl]aMino]Methyl]-5-fluoro-1-(phenylsulfonyl)-, Methyl ester synthesis
- Product Name:1H-Pyrrolo[2,3-b]pyridine-3-carboxylic acid, 2-[[(cyanoMethyl)[(4-Methylphenyl)sulfonyl]aMino]Methyl]-5-fluoro-1-(phenylsulfonyl)-, Methyl ester
- CAS Number:1312755-50-2
- Molecular formula:C25H21FN4O6S2
- Molecular Weight:556.5858
Yield:1312755-50-2 82%
Reaction Conditions:
Stage #1: 1-benzenesulfonyl-5-fluoro-2-bromomethyl-1H-pyrrolo[2,3-b]pyridine-3-carboxylic acid methyl ester;N-(cyanomethyl)-(4-methylphenyl)sulfonamidewith sodium hydride in N,N-dimethyl-formamide;mineral oil at 0 - 20;
Stage #2: with hydrogenchloride in water;N,N-dimethyl-formamide;mineral oil;
Steps:
Step 7 : l-Benzenesulfonyl-5-fluoro-2-{ rcyanomethyl-(toluene-4-sulfonyl)aminol- methyl|-lH-pyrrolor2,3-blpyridine-3-carboxylic acid methyl esterSodium hydride (1.6 g, 60% dispersion in mineral oil, 40.5 mmol) was added portionwise to a cooled (0 °C) solution of l-benzenesulfonyl-5-fluoro-2-bromomethyl-lH-pyrrolo[2,3- b]pyridine-3-carboxylic acid methyl ester (15.8 g, 36.9 mmol) and N-cyanomethyl-4- methyl-benzenesulfonamide (8.5 g, 40.5 mmol) in DMF (150 mL). The reaction mixture was stirred at 0 °C for 15 minutes, then allowed to warm to ambient temperature and stirred for 1 hour. The reaction mixture was poured into a cooled, stirred solution of 2M hydrochloric acid (400 mL). The resultant precipitate was collected by filtration (slow) and the cake washed with water, followed by methanol and then diethyl ether. The resulting cake was dried to afford the title compound as a grey solid (16.7 g, 82%). NMR (400 MHz, DMSO-d6): 8.49 (dd, J = 2.8, 1.2 Hz, 1H), 8.24-8.24 (m, 2H), 8.14 (dd, J = 8.8, 2.8 Hz, 1H), 7.75-7.74 (m, 1H), 7.70-7.68 (m, 2H), 7.62-7.62 (m, 2H), 7.39-7.35 (m, 2H), 5.36 (s, 2H), 4.42 (s, 2H), 3.88 (s, 3H), 2.36 (s, 3H).
References:
WO2011/73263,2011,A1 Location in patent:Page/Page column 86-87
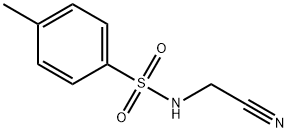
21717-96-4
411 suppliers
$10.00/1g
![1H-Pyrrolo[2,3-b]pyridine-3-carboxylic acid, 2-[[(cyanoMethyl)[(4-Methylphenyl)sulfonyl]aMino]Methyl]-5-fluoro-1-(phenylsulfonyl)-, Methyl ester](/CAS/GIF/1312755-50-2.gif)
1312755-50-2
0 suppliers
inquiry

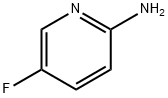
823218-51-5
93 suppliers
$64.00/1g
![1H-Pyrrolo[2,3-b]pyridine-3-carboxylic acid, 2-[[(cyanoMethyl)[(4-Methylphenyl)sulfonyl]aMino]Methyl]-5-fluoro-1-(phenylsulfonyl)-, Methyl ester](/CAS/GIF/1312755-50-2.gif)
1312755-50-2
0 suppliers
inquiry
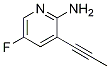
1312755-46-6
0 suppliers
inquiry
![1H-Pyrrolo[2,3-b]pyridine-3-carboxylic acid, 2-[[(cyanoMethyl)[(4-Methylphenyl)sulfonyl]aMino]Methyl]-5-fluoro-1-(phenylsulfonyl)-, Methyl ester](/CAS/GIF/1312755-50-2.gif)
1312755-50-2
0 suppliers
inquiry
![1H-Pyrrolo[2,3-b]pyridine-3-carboxylic acid, 5-fluoro-2-Methyl-, Methyl este](/CAS/20200611/GIF/1312755-47-7.gif)
1312755-47-7
9 suppliers
inquiry
![1H-Pyrrolo[2,3-b]pyridine-3-carboxylic acid, 2-[[(cyanoMethyl)[(4-Methylphenyl)sulfonyl]aMino]Methyl]-5-fluoro-1-(phenylsulfonyl)-, Methyl ester](/CAS/GIF/1312755-50-2.gif)
1312755-50-2
0 suppliers
inquiry
![1H-Pyrrolo[2,3-b]pyridine-3-carboxylic acid, 2-(broMoMethyl)-5-fluoro-1-(phenylsulfonyl)-, Methyl ester](/CAS/GIF/1312755-49-9.gif)