
3-(4,6-Diphenyl-1,3,5-triazin-2-yl)-9-phenyl-9H-carbazole synthesis
- Product Name:3-(4,6-Diphenyl-1,3,5-triazin-2-yl)-9-phenyl-9H-carbazole
- CAS Number:1313391-57-9
- Molecular formula:C33H22N4
- Molecular Weight:474.55
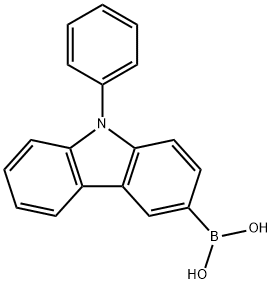
854952-58-2
285 suppliers
$18.50/250mg
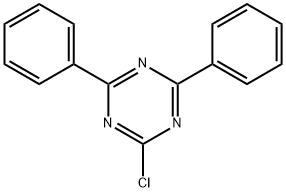
3842-55-5
309 suppliers
$6.00/1g

1313391-57-9
30 suppliers
inquiry
Yield:1313391-57-9 90%
Reaction Conditions:
with potassium carbonate;tetrakis(triphenylphosphine) palladium(0) in tetrahydrofuran;water;toluene; for 9 h;Reflux;
Steps:
1
Example 1Synthesis of Compound Represented by Chemical Formula 9; The compound represented by Chemical Formula 9 as a specific example of an embodiment was synthesized according to the following Reaction Scheme 1. 5 g (0.0135 mol) of material (A), 4.7 g (0.0176 mol) of N-phenyl carbazole boronic acid (material (B)), and 0.78 g (0.7 mmol) of tetrakis(triphenylphosphine)palladium were in suspended in 100 ml of tetrahydrofuran and 100 ml of toluene, and a solution prepared by dissolving 2.8 g (0.02 mol) of potassium carbonate in 100 ml of water was added thereto to form a mixture. The mixture was heated and refluxed for 9 hours. The liquid reactant was separated into two layers, and an organic layer thereof was cleaned with a sodium chloride saturated aqueous solution and dried with anhydrous sodium sulfate.Then, an organic solvent therein was distilled and removed under a reduced pressure, and the residue of the reactant was recrystallized with toluene. The precipitate was filtered and cleaned with toluene, obtaining 8 g of a compound represented byChemical Formula 9 (yield: 90%).
References:
US2012/267620,2012,A1 Location in patent:Page/Page column 14