
4-[1-(N-BOC-AMino)cyclopropyl]phenylboronic acid, pinacol ester synthesis
- Product Name:4-[1-(N-BOC-AMino)cyclopropyl]phenylboronic acid, pinacol ester
- CAS Number:1313441-88-1
- Molecular formula:C20H30BNO4
- Molecular Weight:359.27
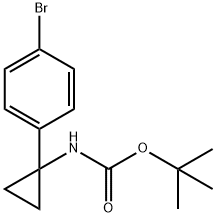
360773-84-8
74 suppliers
$29.00/250mg

73183-34-3
563 suppliers
$6.00/5g
![4-[1-(N-BOC-AMino)cyclopropyl]phenylboronic acid, pinacol ester](/CAS/20150408/GIF/1313441-88-1.gif)
1313441-88-1
21 suppliers
$105.00/50mg
Yield:1313441-88-1 90%
Reaction Conditions:
with (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride;potassium acetate in 1,4-dioxane at 90; for 3 h;Inert atmosphere;
Steps:
2 tert-butyl ( 3 -cyclopropyl-4 -( 4,4, 5,5-tetramethyl-l, 3, 2-dioxaborolan-2-yl )benzyl )carbamate
General procedure: To a stirred solution of tert-butyl (4-bromo-3-cyclopropylbenzyl)carbamate (200 mg, 0.613 mmol) and /Ls(pmacolato)diboron (467 mg, 1.839 mmol) in 1,4-dioxane (5 mL) were added potassium acetate (180 mg, 1.839 mmol) and 1 , 1 ’-/Lsfdiphenylphosphinojfcrrocene-palladium (II) dichloride dichloromethane complex (49 mg, 0.061 mmol) portion- wise at room temperature. The resulting mixture was stirred for 3 h at 90°C under a nitrogen atmosphere. After cooling to room temperature, the resulting mixture was filtered and the filter cake was washed with methanol (3 x 10 mL). The filtrate was concentrated under reduced pressure. The residue was purified by reverse phase flash chromatography with the following conditions: Column: Spherical C18, 20-40 pm, 330 g; Mobile Phase A: water (plus 10 mM ammonium bicarbonate); Mobile Phase B: acetonitrile; Flow rate: 80 mL/min; Gradient: 75%-95% B in 20 min; Detector: UV 254/220 nm. The fractions containing desired product were collected and concentrated under reduced pressure to afford tert-butyl (3-cyclopropyl-4-(4, 4, 5,5-tetramethyl-l, 3, 2-dioxaborolan-2- yl)benzyl)carbamate as a brown solid Yield 120 mg (52%). NMR (400 MHz, DMSO) d 7.53 (d, J = 7.6 Hz, 1H), 7.34 (t, J = 6.4 Hz, 1H), 6.98 (dd, J = 1.6, 7.6 Hz, 1H), 6.69 (d, J = 1.6 Hz, 1H), 4.06 (d, J = 6.4 Hz, 2H), 2.72-2.56 (m, 1H), 1.39 (s, 9H), 1.30 (s, 12H), 0.99-0.90 (m, 2H), 0.65-0.55 (m, 2H). M/z: [ESL] 374 (M+H)+.
References:
WO2022/150316,2022,A1 Location in patent:Paragraph 00236; 00327-00328; 00448-00449
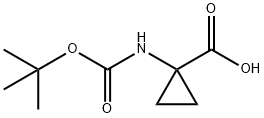
88950-64-5
266 suppliers
$9.00/250mg
![4-[1-(N-BOC-AMino)cyclopropyl]phenylboronic acid, pinacol ester](/CAS/20150408/GIF/1313441-88-1.gif)
1313441-88-1
21 suppliers
$105.00/50mg

24424-99-5
822 suppliers
$13.50/25G
![4-[1-(N-BOC-AMino)cyclopropyl]phenylboronic acid, pinacol ester](/CAS/20150408/GIF/1313441-88-1.gif)
1313441-88-1
21 suppliers
$105.00/50mg
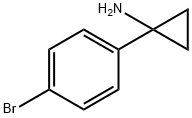
345965-54-0
87 suppliers
$65.00/100mg
![4-[1-(N-BOC-AMino)cyclopropyl]phenylboronic acid, pinacol ester](/CAS/20150408/GIF/1313441-88-1.gif)
1313441-88-1
21 suppliers
$105.00/50mg