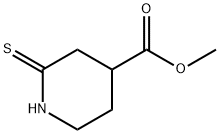
4-Piperidinecarboxylic acid, 2-thioxo-, methyl ester synthesis
- Product Name:4-Piperidinecarboxylic acid, 2-thioxo-, methyl ester
- CAS Number:1313498-22-4
- Molecular formula:C7H11NO2S
- Molecular Weight:173.23
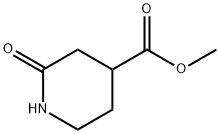
25504-47-6
55 suppliers
$29.00/250mg

1313498-22-4
1 suppliers
inquiry
Yield:1313498-22-4 51%
Reaction Conditions:
with Lawessons reagent in toluene at 100; for 2 h;Inert atmosphere;
Steps:
A Synthesis of methyl 2-thioxopiperidine-4-carboxylate (3):
To a stirred solution of compound 2 (6 g, 38.2 mmol) in toluene (60 mL) was added Lawesson's reagent (7.7 g, 19.1 mmol) under nitrogen atmosphere. The reaction mixture was stirred at 100 °C for 2 h. After consumption of the starting material (by TLC), the reaction mixture was cooled to room temperature and concentrated under reduced pressure. The residue was purified by column chromatography by eluting with 40% EtOAc/n-hexane to obtain compound 3 (3.4 g, 51 %) as a white solid. 1H NMR (500 MHz, DMSO-d6) δ 10.17 (br s, 1H), 3.63 (s, 3H), 3.27-3.21 (m, 2H), 2.95-2.88 (m, 1H), 2.87-2.80 (m, 1H), 2.80-2.72 (m, 1H), 2.05- 1.98 (m, 1H), 1.80-1.70 (m, 1H). LCMS (m/z): 174.1 [M++1].
References:
WO2018/26798,2018,A1 Location in patent:Page/Page column 39; 40
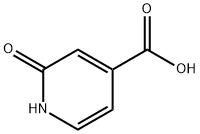
22282-72-0
252 suppliers
$5.00/1g

1313498-22-4
1 suppliers
inquiry
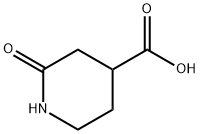
24537-50-6
74 suppliers
$20.00/100mg

1313498-22-4
1 suppliers
inquiry