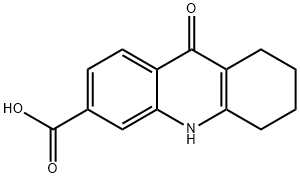
9-oxo-5,6,7,8,8a,9-hexahydroacridine-3-carboxylic acid synthesis
- Product Name:9-oxo-5,6,7,8,8a,9-hexahydroacridine-3-carboxylic acid
- CAS Number:1314749-35-3
- Molecular formula:C14H13NO3
- Molecular Weight:243.26
10312-55-7
283 suppliers
$7.00/1g

108-94-1
527 suppliers
$12.00/50g

1314749-35-3
4 suppliers
$2322.00/5g
Yield:1314749-35-3 98%
Reaction Conditions:
in diphenylether at 250;
Steps:
1
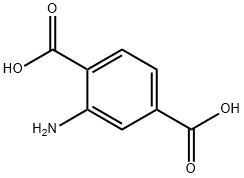
Step 1: condensation; 9-oxo-5, 6, 7, 8, 9, 10-hexahydro-acridine-3-carboxylic acid (VIa); To a suspension of 2-amino terephthalic acid (12 g, 6.6 mmols) in diphenyl ether (120 mL), cyclohexanone (25 mL) was added and the reaction mixture was heated to 250°C for 10 min. Reaction completion was monitored by LC/MS (75 % starting material and 25 % Product formation was observed). Cyclohexanone (25 mL) was added and the reaction mixture was heated to 250°C for another 10 min. (LC/MS showed 50 % product formation). The above process was repeated till LC/MS showed complete product formation (Starting material <2 %). The reaction mixture was cooled to 25 °C, product was filtered, washed with hexane (100 mL) and dried under vacuum to get 15.8 g of (VIa) (98%) as a yellow solid. 1H NMR (300 MHz, DMSO) δ 13.27 (s, 1H), 11.54 (s, 1H), 8.14-8.11 (d, 2H, J = 8.4 Hz), 7.72-7.70 (m, 1H), 2.72 (m, 2H), 2.44 (m, 2H), 1.76-1.72 (m, 4H). MS: calcd for C14H13NO3, 243.09; found 243.8 (M+H)+.
References:
EP2357176,2011,A1 Location in patent:Page/Page column 13-14