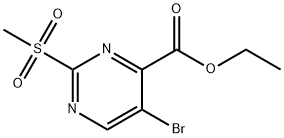
Ethyl 5-bromo-2-(methylsulfonyl)pyrimidine-4-carboxylate synthesis
- Product Name:Ethyl 5-bromo-2-(methylsulfonyl)pyrimidine-4-carboxylate
- CAS Number:1316122-36-7
- Molecular formula:C8H9BrN2O4S
- Molecular Weight:309.14
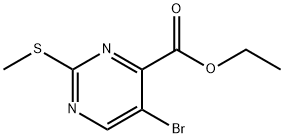
74840-38-3
27 suppliers
$34.00/250mg

1316122-36-7
5 suppliers
inquiry
Yield:1316122-36-7 77%
Reaction Conditions:
with 3-chloro-benzenecarboperoxoic acid in dichloromethane at 0 - 20; for 6.5 h;Inert atmosphere;
Steps:
126.1
A solution of 5-bromo-2-methylthio-pyrimidine-4-carboxylic acid ethyl ester (100 mg, 0.36 mmol ; prepared from 5-bromo-2-methylthio-pyrimidine-4-carboxylic acid according to Coll. Czech. Chem. Commun. 1980, 45(2), 539) in dichloromethane (5 ml) was stirred at 0° C. under argon atmosphere and a solution of 3-chloroperbenzoic acid (178 mg, 0.72 mmol) in dichloromethane (5 ml) was added slowly. After continuous stirring for 30 min and warming-up to r.t., stirring was continued for another 6 h. The reaction mixture was partitioned between dichloromethane and sodium bicarbonate (saturated aqueous solution) and extracted. The organic phase was washed with water, dried and the solvent was evaporated. The product was obtained after purification by silica gel chromatography using a heptane /ethyl acetate gradient as colorless waxy solid (86 mg, 77%). MS: M=309.0 (M+H)+
References:
US2011/306589,2011,A1 Location in patent:Page/Page column 82