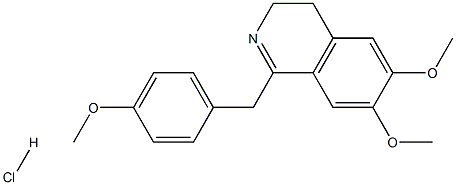
3,4-Dihydro-6,7-diMethoxy-1-(p-Methoxybenzyl)isoquinoline Hydrochloride synthesis
- Product Name:3,4-Dihydro-6,7-diMethoxy-1-(p-Methoxybenzyl)isoquinoline Hydrochloride
- CAS Number:13233-00-6
- Molecular formula:C19H22ClNO3
- Molecular Weight:347.8359
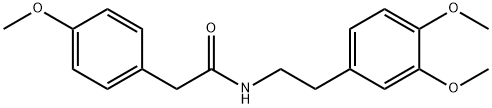
4078-65-3
12 suppliers
inquiry

13233-00-6
8 suppliers
inquiry
Yield:13233-00-6 98%
Reaction Conditions:
with trichlorophosphate in chloroform at 80; for 33 h;Heating / reflux;
Steps:
1.2
step 2) Synthesis of 6, 7-Dimethoxy-l- (p- methoxyphenylmethyl) -3, 4-dihydroisoquinoline hydrochloride salt [scheme 4l In a 500 ml round-bottom flask, N- (3, 4- Dimethoxyphenethyl) (p-methoxyphenyl) acetamide (96 g, 0.291 mol) was dissolved in chloroform (600 mL). POC13 (109 mL, 1.17 mol) was added dropwise thereto, and the flask, equipped with a reflux condenser, was filled with nitrogen gas. Using the thermostat, the reaction solution was adjusted to the temperature of 80 °C and refluxed for 33 hours. Completion of the reaction was detected by thin film chromatography. The chloroform solvent was removed under reduced pressure. The produced light green solid was dissolved in a minimal amount of chloroform, to which distilled ethyl acetate (400 mL) was added, with stirring, to precipitate a solid. Such a solid was filtered via a Buchner funnel, followed by removing the solvent under reduced pressure, to give the desired compound, iminium salt (99.2 g, 98%) as a light-green solid. m. p = 120 °C Rf : 0.38 (hexane : ethyl acetate = 0. 5 : 1) 1H-NMR (300Hz, DMSO): 8 7.57 (s, 1H), 7.39 (d, 2H), 7.12 (s, 1H), 6.92 (d, 2H), 4.41 (s, 2H), 3.82 (s, 3H), 3.79 (s, 3H), 3.64 (s, 3H), 3.01 (t, 2H), 2.43 (s, 2H) HRMs : m/z calcd for ClgHl2NO3 (M+) : 347. 13. Found: 353.55
References:
WO2003/95426,2003,A1 Location in patent:Page/Page column 43-44