
1-(2-Methylbenzoyl)-N-phenyl-1H-indazole-3-carboxamide synthesis
- Product Name:1-(2-Methylbenzoyl)-N-phenyl-1H-indazole-3-carboxamide
- CAS Number:1325681-81-9
- Molecular formula:C22H17N3O2
- Molecular Weight:355.39
Yield:1325681-81-9 20%
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 20;
Steps:
5.1.1. General procedures for 3a-k, 3m, and 3q
General procedure: To a cooled (0 °C) suspension of 1H-indazole-3-carboxylic acid phenylamide 234 (0.42 mmol) and a catalytic amount of Et3N (0.05 mL) in anhydrous CH2Cl2 (1-2 mL), the appropriate aroyl chloride (1.25 mmol) was added. The mixture was stirred for 1-2 h at 0 °C and then for 1-3 h at room temperature. The precipitate was filtered off, and the solvent was evaporated in vacuo. Cold water (20 mL) was added, the mixture was neutralized with 0.5 N NaOH, and the precipitate was recovered by vacuum filtration. For compounds 3c, 3e-f, 3k, and 3q, the reaction mixture was extracted with CH2Cl2 (3 × 15 mL) after dilution. The solvent was dried over sodium sulphate to obtain the desired final compounds. Compound 3c was purified by column chromatography using toluene/ethyl acetate 8:2 as eluent.
References:
Crocetti, Letizia;Giovannoni, Maria Paola;Schepetkin, Igor A.;Quinn, Mark T.;Khlebnikov, Andrei I.;Cilibrizzi, Agostino;Piaz, Vittorio Dal;Graziano, Alessia;Vergelli, Claudia [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 15,p. 4460 - 4472] Location in patent:experimental part
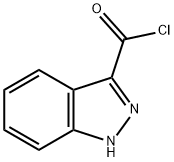
72083-74-0
18 suppliers
$420.61/1gm:

1325681-81-9
3 suppliers
inquiry
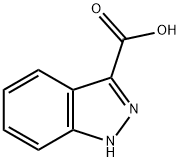
4498-67-3
464 suppliers
$6.00/5g

1325681-81-9
3 suppliers
inquiry

