N-(3-Bromo-2-methyl-6-nitrophenyl)-2,2,2-trifluoroacetamide synthesis
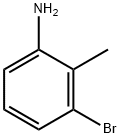
- Product Name:N-(3-Bromo-2-methyl-6-nitrophenyl)-2,2,2-trifluoroacetamide
- CAS Number:1325729-87-0
- Molecular formula:C9H6BrF3N2O3
- Molecular Weight:327.05
Yield:1325729-87-0 74%
Reaction Conditions:
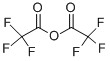
Stage #1: 3-bromo-2-methylaniline;trifluoroacetic anhydride in dichloromethane at 0; for 0.5 h;
Stage #2: with potassium nitrate in dichloromethane at 0 - 20;
Steps:
Intermediate 13; 4-bromo-3-methylbenzene-l,2-diamineIntermediate 13A; A^-(3-bromo-2-methyl-6-nitrop ienyl)-2,2,2-trifluoroacetamide; To a solution of 3-bromo-2-methylaniline (1.0 g, 5.37 mmol) in CH2CI2 (4.0 mL) at 0 °C was added trifluoroacetic anhydride (2.0 mL, 14.2 mmol). The mixture was stirred at 0 °C for 30 minutes, and solid potassium nitrate (0.679 g, 6.72 mmol) was added. The cooling bath was removed, and the mixture was stirred at room temperature overnight. LCMS showed a single product formed. The mixture was concentrated in vacuo, and the residue was partitioned between water and CH2CI2 (2χ). The organic layers were combined and dried over Na2S04. The drying agent was filtered off and the crude product was purified by crystallization from aq EtOH to give the title compound (1.3 g, 74%).
References:
WO2012/83170,2012,A1 Location in patent:Page/Page column 156