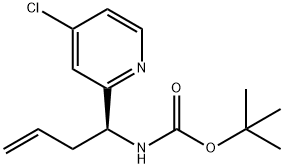
tert-butyl(S)-(1-(4-chloropyridin-2-yl)but-3-en-1-yl)carbamate synthesis
- Product Name:tert-butyl(S)-(1-(4-chloropyridin-2-yl)but-3-en-1-yl)carbamate
- CAS Number:1329171-69-8
- Molecular formula:C14H19ClN2O2
- Molecular Weight:282.77

24424-99-5
842 suppliers
$13.50/25G
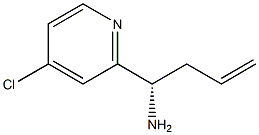
1270210-49-5
7 suppliers
inquiry

1329171-69-8
12 suppliers
inquiry
Yield:1329171-69-8 86%
Reaction Conditions:
with triethylamine in dichloromethane at 20;
Steps:
23D 23D. Preparation of tert-butyl N- [(15)-i -(4-chloropyridin-2-yl)but-3 -en- i-yl] carbamate
(15)-i -(4-Chloropyridin-2-yl)but-3 -en-i-amine (42g, 230 mmol) was dissolved inDCM (420 mL), Et3N (32. i mL, 230 mmol) was added followed by dropwise addition ofBOC2O (53.4 mL, 230 mmol). The reaction was stirred at rt for 2-3 h. The reaction was diluted with excess DCM (i L), washed with water (500 ml) and brine (500m1). The organic layer was dried over Na2SO4, filtered, and concentrated. The crude product was purified using silica gel chromatography to give tert-butyl N-[( i 5)-i -(4-chloropyridin-2- yl)but-3-en-i-yl]carbamate (6i g, 86%) as a pale yellow solid. MS(ESI) m/z: 283.2(M+H). ‘H NMR (500 MHz, CDC13) ? 8.44 (d, iH), 7.26-7.i6 (dd, 2H), 5.69-5.6i (m, iH), 5.59 (bs, iH), 5.07-5.03 (m, 2H), 4.76 (bs, iH), 2.62-2.55 (m, 2H), i.42 (s, 9H).
References:
WO2015/116886,2015,A1 Location in patent:Page/Page column 184; 186

24424-99-5
842 suppliers
$13.50/25G
![2-Propanesulfinamide, N-[(1S)-1-(4-chloro-2-pyridinyl)-3-buten-1-yl]-2-methyl-, [S(S)]-](/CAS/20210305/GIF/1329167-87-4.gif)
1329167-87-4
0 suppliers
inquiry

1329171-69-8
12 suppliers
inquiry
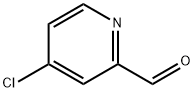
63071-13-6
136 suppliers
$75.00/1g

1329171-69-8
12 suppliers
inquiry
![2-Propanesulfinamide, N-[(4-chloro-2-pyridinyl)methylene]-2-methyl-, [N(E),S(S)]-](/CAS/20240320/GIF/1329167-86-3.gif)
1329167-86-3
0 suppliers
inquiry

1329171-69-8
12 suppliers
inquiry
![2-Propanesulfinamide, N-[(1S)-1-(4-chloro-2-pyridinyl)-3-buten-1-yl]-2-methyl-, [S(S)]-](/CAS/20210305/GIF/1329167-87-4.gif)
1329167-87-4
0 suppliers
inquiry

1329171-69-8
12 suppliers
inquiry