
3-[(4-Chlorophenyl)thio]-1-(diphenylmethyl)azetidine synthesis
- Product Name:3-[(4-Chlorophenyl)thio]-1-(diphenylmethyl)azetidine
- CAS Number:132924-59-5
- Molecular formula:C22H20ClNS
- Molecular Weight:365.92
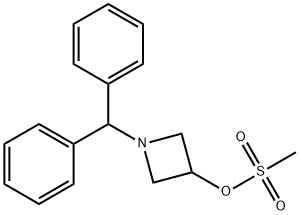
33301-41-6
229 suppliers
$13.50/250mg

106-54-7
250 suppliers
$10.00/5g
![3-[(4-Chlorophenyl)thio]-1-(diphenylmethyl)azetidine](/CAS/GIF/132924-59-5.gif)
132924-59-5
9 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: p-Chlorothiophenolwith sodium hydride in DMF (N,N-dimethyl-formamide) at 20; for 0.166667 h;
Stage #2: 1-benzhydryl-3-azetidinyl methanesulfonate in DMF (N,N-dimethyl-formamide) at 60; for 5 h;
Steps:
19.a
A solution of 4-chlorothiophenol (3.0 g, 20.7 mmol) in dimethylformamide (30 ml) is treated with 60% sodium hydride dispersion in mineral oil (1.2 g, 30 mmol), stirred for 10 minutes at ambient temperature, then treated with a solution of methanesulfonic acid 1-benzhydryl- azetidin-3-yl ester (5.98 g, 18. 8 mmol) in dimethylformamide (40 ml). The reaction mixture is heated at 60°C for 5 hours, then cooled to ambient temperature and partitioned between ethyl acetate and water. The organic phase is dried over magnesium sulphate and evaporated. The crude product is purified by flash silica chromatography (elution with iso-hexane: ether, 8: 2) to afford 1-BENZHYDRYL-3- (4-CHLORO-PHENYLSULFANYL)-AZETIDINE as a pale yellow solid.
References:
WO2005/26113,2005,A1 Location in patent:Page 38
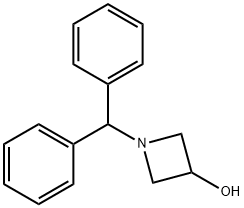
18621-17-5
380 suppliers
$5.00/1g
108-20-3
565 suppliers
$25.00/25mL

6153-56-6
414 suppliers
$10.00/5g
124-63-0
6 suppliers
$10.00/5g

106-54-7
250 suppliers
$10.00/5g
![3-[(4-Chlorophenyl)thio]-1-(diphenylmethyl)azetidine](/CAS/GIF/132924-59-5.gif)
132924-59-5
9 suppliers
inquiry