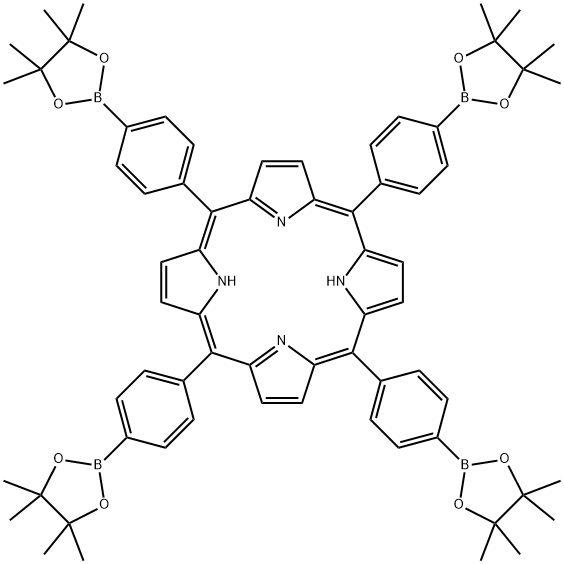
5,1O,15,20-tetrakis-[4-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-phenyl]-porphyrin synthesis
- Product Name:5,1O,15,20-tetrakis-[4-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-phenyl]-porphyrin
- CAS Number:1332748-00-1
- Molecular formula:C68H74B4N4O8
- Molecular Weight:1118.58
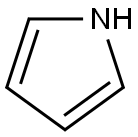
109-97-7
339 suppliers
$9.00/5 g
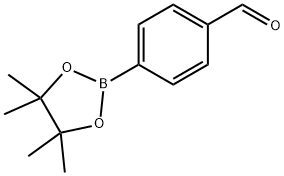
128376-64-7
179 suppliers
$5.00/250mg
![5,1O,15,20-tetrakis-[4-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-phenyl]-porphyrin](/CAS/20210111/GIF/1332748-00-1.gif)
1332748-00-1
10 suppliers
inquiry
Yield: 13%
Reaction Conditions:
Stage #1:pyrrole;4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzaldehyde with boron trifluoride diethyl etherate in chloroform at 20; for 2 h;
Stage #2: with chloranil in chloroform at 20; for 1 h;
Steps:
Scheme I[ 00189 ] 5, 10,15 , 20-Tetrakis- [4- (4 , 4 , 5 , 5-tetra ethyl- [1 , 3,2]dioxaborolan-2-yl) -phenyl] -porphyrin (Intermediate 1) 3 : Under an atmosphere of Argon, BF3 -Et20 (1.0 mL) was added to a solution of pyrrole 1 (1.8 mL, 26.0 mraol) and aldehyde 2 (6.0 g, 26.0 mmol ) dissolved in chloroform (900 mL) . After stirring for 2 h at ambient temperature, p-chloranil (10.5 g, 42 mmol) was added. The mixture was stirred at ambient temperature for 1 hour, and then triethylamine (2 mL) was added to quench BF3-Et20. The mixture was passed through a bed of silica on a sintered Biichner funnel, and then washed with chloroform until the filtrate appeared colorless. After the filtrate was concentrated in vacuo, the resulting crude solid was triturated with excess methanol, filtered, and washed thoroughly with methanol (500 mL) to afford compound 3 as a purple solid (3.8 g, yield = 13%) *H NMR (500 MHz, CD2C12, 298 K) : δ 8.85 (s, 8H, pyrrole-H) , 8.23 (AB q, JAB= 8.0 Hz, 10.0 Hz, 16H, Ar-H), 1.53 (s, 48H, Me-H) . 13C NMR (125 MHz, CD2C12, 298 K) : δ 145.08, 135.11, 133.03, 120.12, 84.14, ]77.25, 25.08. HRMS-ESI : Calculated for C68H74B4N408 [M + H]+ ml z = 1119.5957, found ml z = 1119.6047.
References:
THE REGENTS OF THE UNIVERSITY OF CALIFORNIA;YAGHI, Omar, M.;WAN, Shun;DOONAN, Christian, J.;WANG, Bo;DENG, Hexiang WO2012/82213, 2012, A2 Location in patent:Page/Page column 69-70