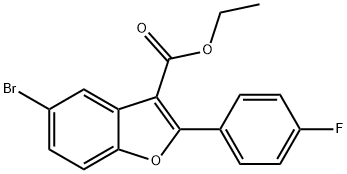
ethyl 5-bromo-2-(4-fluorophenyl)benzofuran-3-carboxylate synthesis
- Product Name:ethyl 5-bromo-2-(4-fluorophenyl)benzofuran-3-carboxylate
- CAS Number:1333340-13-8
- Molecular formula:C17H12BrFO3
- Molecular Weight:363.18
Yield:1333340-13-8 14.3%
Reaction Conditions:
with di-tert-butyl peroxide;iron(III) chloride hexahydrate in 1,2-dichloro-ethane; for 6 h;Inert atmosphere;Reflux;
Steps:
Synthesisof Compound 4
A mixture of compound 3 (130 g, 0.6 mol), 4-bromophenol (311 g, 1.8 mol) andFeCl3·6H2O (19.5 g, 0.09 mol) in DCE (700 mL) wasstirred at RT, and then 2-(tert-butylperoxy)-2-methylpropane (193g, 1.32 mol) was added drop wise under nitrogen. After heated to reflux and stirred 6 hours,the mixture was cooled to RT, quenched with saturated NaHSO3, andextracted with DCM. The organic phaseswere washed with water, brine and dried over Na2SO4, filtered and evaporated.The residue was purified by column chromatography (PE : DCM = 15 : 1) to give thecrude product, which was crystallizedfrom cold MeOHto afford compound 4 (37 g, yield: 14.3%) as solid. 1H-NMR (CDCl3, 400 MHz) d 8.12 (s, 1H), 7.97~8.01 (m, 2H), 7.37 (d, J = 4.0 Hz, 1H), 7.32 (d, J =8.0 Hz, 1H), 7.11 (t, J = 8.0 Hz,2H), 4.32~4.38 (m, 2H), 1.36 (t, J =8.0 Hz, 3H).
References:
He, Shuwen;Li, Peng;Dai, Xing;McComas, Casey C.;Du, Chunyan;Wang, Ping;Lai, Zhong;Liu, Hong;Yin, Jingjun;Bulger, Paul G.;Dang, Qun;Xiao, Dong;Zorn, Nicolas;Peng, Xuanjia;Nargund, Ravi P.;Palani, Anandan [Tetrahedron Letters,2014,vol. 55,# 14,p. 2212 - 2216] Location in patent:supporting information
403-42-9
527 suppliers
$5.00/10g

1333340-13-8
7 suppliers
inquiry