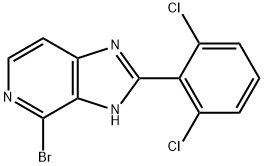
4-broMo-2-(2,6-dichlorophenyl)-1H-iMidazo[4,5-c]pyridine synthesis
- Product Name:4-broMo-2-(2,6-dichlorophenyl)-1H-iMidazo[4,5-c]pyridine
- CAS Number:1334411-79-8
- Molecular formula:C12H6BrCl2N3
- Molecular Weight:343.01
![4-Chloro-2-(2,6-dichlorophenyl)-3H-iMidazo[4,5-c]pyridine](/CAS/GIF/1334411-81-2.gif)
1334411-81-2
13 suppliers
$247.00/250mg
![4-broMo-2-(2,6-dichlorophenyl)-1H-iMidazo[4,5-c]pyridine](/CAS/GIF/1334411-79-8.gif)
1334411-79-8
20 suppliers
$64.00/100mg
Yield:1334411-79-8 70%
Reaction Conditions:
Stage #1: 4-chloro-2-(2,6-dichlorophenyl)-1H-imidazo[4,5-c]pyridinewith hydrogen bromide;acetic acid at 90;
Stage #2: with potassium carbonate in water at 20; pH=7;Product distribution / selectivity;Cooling with ice;
Steps:
I.A.2
2-(2,6-dichlorophenyl)-lH-imidazo[4,5-c]pyridin-4-ol (0.2 g, 0.6 mmol) and a HBr solution in AcOH (33%, 2 mL) was heated at 90 °C overnight. The reaction was cooled to room temperature and poured into ice followed by addition of an aqueous K2CC"3 to neutralize to pH 7.0. The mixture was extracted with EtOAc (3 x 50 mL), washed with brine, dried over Na2S04. Concentration via rotavap gave desired product as a pale yellow solid (0.14 g, 70% yield). NMR (DMSO- , 500 MHz): δ 13.78 (s, 1H), 8.18 (m, 1H), 7.69 (m, 4H). LCMS(ESI) m/z: 343 [M+H+]
References:
WO2011/113802,2011,A2 Location in patent:Page/Page column 103-104
![2-(2,6-DICHLOROPHENYL)-1H-IMIDAZO[4,5-C]PYRIDIN-4-OL](/CAS/20180601/GIF/1334411-80-1.gif)
1334411-80-1
5 suppliers
inquiry
![4-broMo-2-(2,6-dichlorophenyl)-1H-iMidazo[4,5-c]pyridine](/CAS/GIF/1334411-79-8.gif)
1334411-79-8
20 suppliers
$64.00/100mg
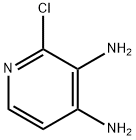
39217-08-8
193 suppliers
$18.00/1g
![4-broMo-2-(2,6-dichlorophenyl)-1H-iMidazo[4,5-c]pyridine](/CAS/GIF/1334411-79-8.gif)
1334411-79-8
20 suppliers
$64.00/100mg
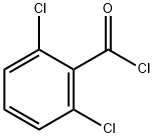
4659-45-4
270 suppliers
$12.00/5g
![4-broMo-2-(2,6-dichlorophenyl)-1H-iMidazo[4,5-c]pyridine](/CAS/GIF/1334411-79-8.gif)
1334411-79-8
20 suppliers
$64.00/100mg

83-38-5
447 suppliers
$6.00/10g
![4-broMo-2-(2,6-dichlorophenyl)-1H-iMidazo[4,5-c]pyridine](/CAS/GIF/1334411-79-8.gif)
1334411-79-8
20 suppliers
$64.00/100mg