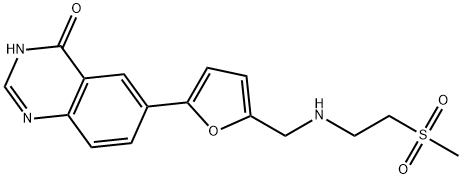
6-(5-((2-(Methylsulfonyl)ethylaMino)Methyl)furan-2-yl)quinazolin-4(3h)-one synthesis
- Product Name:6-(5-((2-(Methylsulfonyl)ethylaMino)Methyl)furan-2-yl)quinazolin-4(3h)-one
- CAS Number:1334953-74-0
- Molecular formula:C16H17N3O4S
- Molecular Weight:347.39
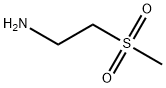
49773-20-8
58 suppliers
$41.00/250mg
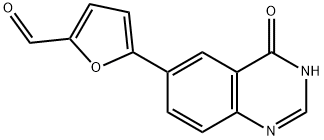
1334953-71-7
4 suppliers
inquiry

1334953-74-0
10 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 2-Methanesulfonyl-ethylamine;5-(4-oxo-3,4-dihydroquinazolin-6-yl)furan-2-carbaldehydewith acetic acid;N-ethyl-N,N-diisopropylamine in tetrahydrofuran at 35; for 2.5 h;
Stage #2: with sodium tris(acetoxy)borohydride in tetrahydrofuran at 20;
Steps:
7
Example 7: Synthesis of IM4 (6-(5-((2-(methylsulfonyl)ethylatnino)methyl)furan-2- quinazolin-^S/ -one)To a suspension of IM1 (5 g, 21 mmol) and 2-(methylsulfonyl)ethanamine (4.1 g, 33 mmol) in THF (150 mL) was added acetic acid (5 g, 83 mmol) followed by DIPEA (10.5 g, 83 mmol). The mixture was stirred at 35°C (internal temperature) for 2.5 h and was then cooled to 20°C (internal temperature). Sodium in-acetoxyborohydride (8.8 g, 42 mmol) was added and the mixture was stirred at ambient temperature the reaction was complete (TLC analysis). 25% Aqueous sodium hydroxide (10 mL) and water (50 mL) were added and the mixture was stirred for 30 min. The liquid phases were separated and the aqueous layer was extracted with THF (50 mL). The extract was combined with the former organic layer and the mixture was washed with saturated aqueous NH4C1 (50 mL). The organic layer was concentrated under vacuum. The crude product was purified by column chromatography, eluting with 5% MeOH in DCM providing IM4 (3.1 g) after evaporation of the product containing fractions. IM4: .H NMR (300 MHz, d6-DMSO): δ 2.94 (dt, / = 13.8 Hz, / = 7.05 Hz, 1H), 3.00 (s, 1H), 3.24 (t, J = 6.75 Hz, 1H), 3.77 (s, 1H), 6.42 (d, J = 3.3 Hz, 1H), 7.03 (d, / = 3.0 Hz, 1H), 7.68 (d, = 8.4 Hz, 1H), 8.06 (s, 1H), 8.09 (dd, = 8.55 Hz, J = 2.25 Hz, 1H), 8.31 (d, 7 = 2.1 Hz, 1H).
References:
WO2011/116634,2011,A1 Location in patent:Page/Page column 18