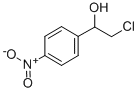
Benzenemethanol, alpha-(chloromethyl)-4-nitro- (9CI) synthesis
- Product Name:Benzenemethanol, alpha-(chloromethyl)-4-nitro- (9CI)
- CAS Number:13407-16-4
- Molecular formula:C8H8ClNO3
- Molecular Weight:201.61
Yield:13407-16-4 86%
Reaction Conditions:
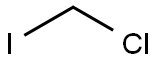
Stage #1: Chloroiodomethanewith TurboGrignard in tetrahydrofuran at -78; for 0.166667 h;Inert atmosphere;
Stage #2: 4-nitrobenzaldehdye in tetrahydrofuran at -78 - 20; for 0.5 h;Inert atmosphere;
Steps:
General procedure: i-PrMgCl LiCl (0.95 mol/L, 1.3 mL, 1.2 mmol) was added to a solution of chloroiodomethane (0.17 mL, 2.4 mmol) in THF (2 mL) under nitrogen atmosphere at 78 C, and the reaction mixture was stirred for 10 min. After this time a solution of benzaldehyde (0.11 g, 1 mmol) in THF (1 mL) was added to the reaction, and the mixture was stirred for 30 min. After that, NH4Cl was added (30 mL) and the reaction products were subjected to ethyl acetate extraction (3 30 mL). The combined organic extracts were dried over anhydrous MgSO4 and the solvent was evaporated under reduced pressure. The residue was purified by column chromatography on silica gel using hexane/ethyl acetate (4:1) as eluent, affording the desired product 4a in 90% yield (light yellow oil).
References:
Nishimura, Rodolfo H.V.;Toledo, Fabiano T.;Lopes, Jo?o L.C.;Clososki, Giuliano C. [Tetrahedron Letters,2013,vol. 54,# 4,p. 287 - 290]
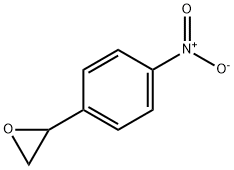
6388-74-5
36 suppliers
inquiry

13407-16-4
9 suppliers
inquiry
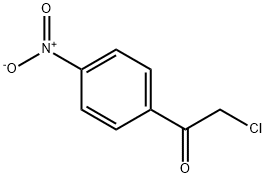
34006-49-0
22 suppliers
inquiry

13407-16-4
9 suppliers
inquiry