
1H-Imidazole, 2-bromo-1-[[2-(trimethylsilyl)ethoxy]methyl]- synthesis
- Product Name:1H-Imidazole, 2-bromo-1-[[2-(trimethylsilyl)ethoxy]methyl]-
- CAS Number:134183-57-6
- Molecular formula:C9H17BrN2OSi
- Molecular Weight:277.23
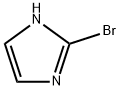
16681-56-4
214 suppliers
$8.00/100mg
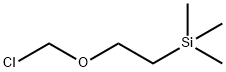
76513-69-4
349 suppliers
$6.00/1g
![1H-Imidazole, 2-bromo-1-[[2-(trimethylsilyl)ethoxy]methyl]-](/CAS/20180529/GIF/134183-57-6.gif)
134183-57-6
2 suppliers
inquiry
Yield:134183-57-6 88.41%
Reaction Conditions:
Stage #1: 2-bromo-1H-imidazolewith sodium hydride in tetrahydrofuran;mineral oil at 0; for 0.5 h;
Stage #2: (2-trimethylethylsilylethoxy)methyl chloride in tetrahydrofuran;mineral oil at 0 - 20; for 15.5 h;
Steps:
45.2 [335] Step 2: 2-[(2-bromoimidazol-1-yl)methoxy]ethyltrimethylsilane
To a solution of 2-bromo-1H-imidazole (300.00 mg, 2.04 mmol, 1.00 eq) in THF (5.00 mL) was added batchwise NaH (106.00 mg, 2.65 mmol, 60% purity, 1.30 eq) at 0C. After addition, the mixture was stirred at this temperature 0.5 h, and then 2-(chloromethoxy) ethyltrimethylsilane (442.14 mg, 2.65 mmol, 470.36 uL, 1.30 eq) was added dropwise at 0C. The resulting mixture was stirred at 20C for 15.5 h. It was quenched by addition water 50 mL, and extracted with EtOAc (20 mLx3), dried over Na2SO4, filtered and concentrated under reduced pressure to give a residue. The residue was purified by column chromatography (SiO2, PE/EtOAc =20/1 to 5:1) to afford the title compound (500.00 mg, 1.80 mmol, 88.41% yield) as a colorless oil.
References:
WO2018/13867,2018,A1 Location in patent:Paragraph 335
![1H-Imidazole, 1-[[2-(trimethylsilyl)ethoxy]methyl]-](/CAS/20210111/GIF/101226-33-9.gif)
101226-33-9
2 suppliers
inquiry
![1H-Imidazole, 2-bromo-1-[[2-(trimethylsilyl)ethoxy]methyl]-](/CAS/20180529/GIF/134183-57-6.gif)
134183-57-6
2 suppliers
inquiry

76513-69-4
349 suppliers
$6.00/1g
![1H-Imidazole, 2-bromo-1-[[2-(trimethylsilyl)ethoxy]methyl]-](/CAS/20180529/GIF/134183-57-6.gif)
134183-57-6
2 suppliers
inquiry