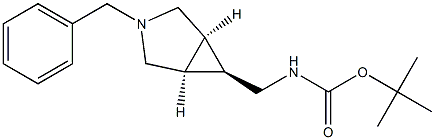
Tert-Butyl (((Meso-1R,5S,6S)-3-Benzyl-3-Azabicyclo[3.1.0]Hexan-6-Yl)Methyl)Carbamate synthesis
- Product Name:Tert-Butyl (((Meso-1R,5S,6S)-3-Benzyl-3-Azabicyclo[3.1.0]Hexan-6-Yl)Methyl)Carbamate
- CAS Number:134575-11-4
- Molecular formula:C18H26N2O2
- Molecular Weight:302.4113

24424-99-5
819 suppliers
$13.50/25G
![(3-benzyl-3-azabicyclo[3.1.0]hexan-6-yl)methanamine](/CAS/20200401/GIF/134575-10-3.gif)
134575-10-3
3 suppliers
inquiry
![Tert-Butyl (((Meso-1R,5S,6S)-3-Benzyl-3-Azabicyclo[3.1.0]Hexan-6-Yl)Methyl)Carbamate](/CAS/20180702/GIF/134575-11-4.gif)
134575-11-4
10 suppliers
inquiry
Yield:134575-11-4 84%
Reaction Conditions:
with dmap in dichloromethane; for 16 h;
Steps:
B.4
Step 4: The crude amine (h) prepared in step 3 and N, N-dimethylaminopyridine (DMAP) (0.66 g, 5.4 mmol) were dissolved in CH2CI2(100ml_). To this solution was added di-tert-butyldicarbonate (7 g, 33 mmol), inportions, and the reaction mixture was stirred for 16 h. The reaction mixture wasthen washed two times (50 ml_) with water and once with brine (50 ml_). Theorganic phase was isolated and dried, and the solvent was removed underreduced pressure. The crude product was subjected to silica gel chromatographyusing 1:3 ethyl acetate:hexane as the eluting solvent. The eluted fractions werecombined and concentrated to yield 6.9 g of pure carbamate (i) (84%).
References:
WO2006/2133,2006,A1 Location in patent:Page/Page column 51-52
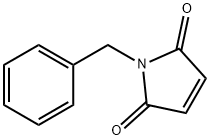
1631-26-1
320 suppliers
$6.00/1g
![Tert-Butyl (((Meso-1R,5S,6S)-3-Benzyl-3-Azabicyclo[3.1.0]Hexan-6-Yl)Methyl)Carbamate](/CAS/20180702/GIF/134575-11-4.gif)
134575-11-4
10 suppliers
inquiry
![3-Azabicyclo[3.1.0]hexane-6-carboxaldehyde, 3-(phenylmethyl)-, (1α,5α,6α)- (9CI)](/CAS/20211123/GIF/134575-08-9.gif)
134575-08-9
2 suppliers
inquiry
![Tert-Butyl (((Meso-1R,5S,6S)-3-Benzyl-3-Azabicyclo[3.1.0]Hexan-6-Yl)Methyl)Carbamate](/CAS/20180702/GIF/134575-11-4.gif)
134575-11-4
10 suppliers
inquiry
![exo-3-Benzyl-3-azabicyclo[3.1.0]hexane-6-methanol](/CAS2/GIF/134575-07-8.gif)
134575-07-8
74 suppliers
$36.00/1g
![Tert-Butyl (((Meso-1R,5S,6S)-3-Benzyl-3-Azabicyclo[3.1.0]Hexan-6-Yl)Methyl)Carbamate](/CAS/20180702/GIF/134575-11-4.gif)
134575-11-4
10 suppliers
inquiry

134575-06-7
51 suppliers
$15.00/250mg
![Tert-Butyl (((Meso-1R,5S,6S)-3-Benzyl-3-Azabicyclo[3.1.0]Hexan-6-Yl)Methyl)Carbamate](/CAS/20180702/GIF/134575-11-4.gif)
134575-11-4
10 suppliers
inquiry