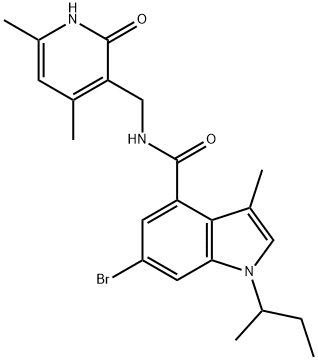
1H-Indole-4-carboxaMide,6-broMo-N-[(1,2-dihydro-4,6-diMethyl-2-oxo-3-pyridinyl)Methyl]-3-Methyl-1-(1-Methylpropyl)- synthesis
- Product Name:1H-Indole-4-carboxaMide,6-broMo-N-[(1,2-dihydro-4,6-diMethyl-2-oxo-3-pyridinyl)Methyl]-3-Methyl-1-(1-Methylpropyl)-
- CAS Number:1346574-53-5
- Molecular formula:C22H26BrN3O2
- Molecular Weight:444.36
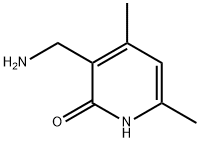
771579-27-2
108 suppliers
$17.00/250mg

1346575-54-9
21 suppliers
inquiry
![1H-Indole-4-carboxaMide,6-broMo-N-[(1,2-dihydro-4,6-diMethyl-2-oxo-3-pyridinyl)Methyl]-3-Methyl-1-(1-Methylpropyl)-](/CAS/20150408/GIF/1346574-53-5.gif)
1346574-53-5
9 suppliers
inquiry
Yield:1346574-53-5 87%
Reaction Conditions:
with 4-methyl-morpholine;1-hydroxy-7-aza-benzotriazole;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride in dimethyl sulfoxide at 20; for 32 h;Inert atmosphere;Sealed tube;
Steps:
266
Example 266 6-bromo- 1 -(sec-butyl)-N-((4,6-dimethyl-2-oxo- 1 ,2-dihydropyridin-3-yl)methyl)-3-methyl- lH-indole-4-carboxamide Added sequentially to a reaction flask were 6-bromo- l-(sec-butyl)-3 -methyl- 1H- indole-4-carboxylic acid (1.33 g, 4.29 mmol), 3-(aminomethyl)-4,6-dimethyl-2(lH)- pyridinone (1.213 g, 6.43 mmol), l-hydroxy-7-azabenzotriazole (0.875 g, 6.43 mmol), EDC (1.233 g, 6.43 mmol), followed by DMSO (30 mL, via syringe) and then N- methylmorpholine (1.886 mL, 17.15 mmol, via syringe). The contents were sealed and stirred at room temperature and the solids gradually dissolved. The contents were stirred at room temperature for 32 h, and then slowly diluted into 220 mL of ice-water with stirring. The contents were stirred for 10 min, and then allowed to stand for an additional 10 min. The contents were filtered and the filtered solid was washed with additional water (50 mL). The solid was then air dried for 10 min, and then in a vacuum oven at 50 °C for 23 h total. The product was collected as 1.75 g (87%). 1H NMR (400 MHz, DMSO- 6) δ ppm 0.69 (t, J=7.33 Hz, 3H), 1.36 (d, J=6.57 Hz, 3H), 1.77 (dq, J=10.29, 7.09 Hz, 2H), 2.12 (d, J=9.09 Hz, 6H), 2.21 (s, 3H), 4.30 (d, J=5.05 Hz, 2H), 4.43 - 4.56 (m, 1H), 5.86 (s, 1H), 6.99 (d, J=1.52 Hz, 1H), 7.30 (s, 1H), 7.77 (d, J=1.77 Hz, 1H), 8.25 (t, J=4.93 Hz, 1H), 11.49 (br. s 1H); LCMS = 444.1 (MH+).
References:
WO2011/140324,2011,A1 Location in patent:Page/Page column 81

1346576-37-1
21 suppliers
inquiry
![1H-Indole-4-carboxaMide,6-broMo-N-[(1,2-dihydro-4,6-diMethyl-2-oxo-3-pyridinyl)Methyl]-3-Methyl-1-(1-Methylpropyl)-](/CAS/20150408/GIF/1346574-53-5.gif)
1346574-53-5
9 suppliers
inquiry
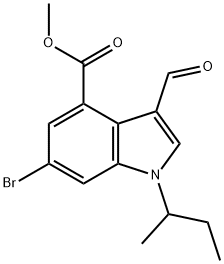
1346576-38-2
13 suppliers
inquiry
![1H-Indole-4-carboxaMide,6-broMo-N-[(1,2-dihydro-4,6-diMethyl-2-oxo-3-pyridinyl)Methyl]-3-Methyl-1-(1-Methylpropyl)-](/CAS/20150408/GIF/1346574-53-5.gif)
1346574-53-5
9 suppliers
inquiry
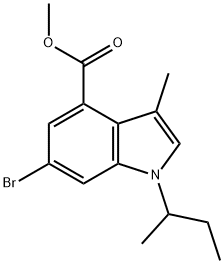
1346576-39-3
9 suppliers
inquiry
![1H-Indole-4-carboxaMide,6-broMo-N-[(1,2-dihydro-4,6-diMethyl-2-oxo-3-pyridinyl)Methyl]-3-Methyl-1-(1-Methylpropyl)-](/CAS/20150408/GIF/1346574-53-5.gif)
1346574-53-5
9 suppliers
inquiry
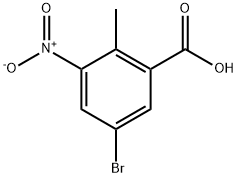
107650-20-4
158 suppliers
$9.00/250mg
![1H-Indole-4-carboxaMide,6-broMo-N-[(1,2-dihydro-4,6-diMethyl-2-oxo-3-pyridinyl)Methyl]-3-Methyl-1-(1-Methylpropyl)-](/CAS/20150408/GIF/1346574-53-5.gif)
1346574-53-5
9 suppliers
inquiry