
Ethyl 1-(2-chloropyrimidin-4-yl)piperidine-3-carboxylate synthesis
- Product Name:Ethyl 1-(2-chloropyrimidin-4-yl)piperidine-3-carboxylate
- CAS Number:1347757-99-6
- Molecular formula:C12H16ClN3O2
- Molecular Weight:269.73
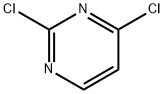
3934-20-1
709 suppliers
$9.00/1g

71962-74-8
48 suppliers
inquiry

1347757-99-6
8 suppliers
$130.78/250mgs:
Yield: 80%
Reaction Conditions:
with triethylamine in ethanol at 0 - 20; for 1 h;
Steps:
1
ETHYL l-(2-CHLOROPYRIMIDIN-4-YL)PIPERIDINE-3-CARBOXYLATE (Ml.l). To a solution of 2,4-dichloropyrimidine (Alfa Aesar, 10 g, 65.8 mmol) and TEA (Sigma-Aldrich, 9.15 mL, 65.8 mmol) in EtOH (68.5 mL) at 0 °C was added ethyl piperidine-3-carboxylate (Sigma-Aldrich, 10.64 mL, 65.8 mmol) in EtOH (13.70 mL). The reaction was then allowed to warm to room temperature while stirring. After 1 hour, the reaction was concentrated and the residue was purified by chromatography through a 340g silica gel column, eluting with 10% to 80 %EtOAc/hexanes, to provide ethyl l-(2-chloropyrimidin-4-yl)piperidine-3-carboxylate as a colorless oil in 80% yield. lH NMR (300 MHz, CDC13) δ ppm 0.94 (t, J=6.94 Hz, 3 H) 1.26 (d, J=9.35 Hz, 1 H) 1.38 - 1.62 (m, 2 H) 1.66 - 1.85 (m, 1 H) 2.26 (m, 1 H) 2.90 - 3.20 (m, 2 H) 3.64 (m, 1 H) 3.69 - 4.09 (m, 3 H) 6.23 (d, J=5.85 Hz, 1 H) 7.68 (d, J=5.99 Hz, 1 H); ESI-MS M+H 270.0.
References:
AMGEN INC.;BRYAN, Marian, C.;CHENG, Alan, C.;DOHERTY, Elizabeth, M.;FALSEY, James, R.;FELLOWS, Ingrid, M.;KIM, Joseph, L.;LEWIS, Richard, T.;POTASHMAN, Michele, H.;WHITTINGTON, Douglas, A. WO2011/143033, 2011, A1 Location in patent:Page/Page column 101