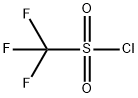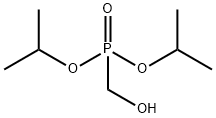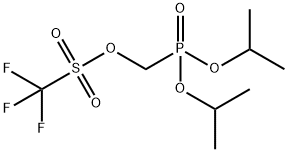
Methanesulfonic acid, 1,1,1-trifluoro-, [bis(1-methylethoxy)phosphinyl]methyl ester synthesis
- Product Name:Methanesulfonic acid, 1,1,1-trifluoro-, [bis(1-methylethoxy)phosphinyl]methyl ester
- CAS Number:134963-85-2
- Molecular formula:C8H16F3O6PS
- Molecular Weight:328.24
Yield:134963-85-2 96%
Reaction Conditions:
Stage #1: diisopropyl hydroxymethylphosphonatewith n-butyllithium in diethyl ether at -78; for 0.0833333 h;
Stage #2: trifluoromethane sulfonyl chloride in diethyl ether at -78; for 1.08333 h;
Steps:
4 Example 4: Synthesis of Diisopropylphosphonomethyl Trifluoromethanesulfonate
This compound was synthesized according to a known procedure: Dumbre, S. G., Jang, M., Herdewijn, P. J. Org. Chem.2013, 78, 7137-7144. To a solution of (hydroxymethyl)diisopropylphosphonate (10.0 g, 50.97 mmol) in 170 mL of dry diethyl ether at-78°C was added a solution of 1.6 M n-BuLi (35.04 mL, 56.07 mmol). The reaction mixture was allowed to stir at this temperature for 5 min and trifluoromethanesulfonyl chloride (5.97 mL, 56.07 mmol) was added dropwise over 5 minutes. The reaction mixture was stirred at this temperature for 1 h. and was quenched with sat. NH4Cl. The organic layer was washed with brine, dried over NaSO4, and concentrated under reduced pressure at rt gave diisopropylphosphonomethyl triflate (16.1 g, 96% yield). 1H NMR (300 MHz, CDCl3): δ 4.774.88 [m, 2H, CH(CH3)2], 4.56 (d, 1JP,H = 8.98 Hz, 2H, PCH2), 1.36-1.40 [m, 12H, CH(CH3)2]; 13C NMR (75 MHz, CDCl3): δ 118.4 (d, 1JC,F = 318.8 Hz, CF3), 73.0 [d, 2JP,C = 6.7 Hz, CH(CH3)2], 67.0 (d, 2JP,C = 169.8 Hz, PCH2), 23.7 [CH(CH3)2]. 31P NMR (121 MHz, CDCl3): δ 10.0
References:
WO2016/174081,2016,A1 Location in patent:Page/Page column 22; 23
1809-20-7
174 suppliers
$8.00/10g
![Methanesulfonic acid, 1,1,1-trifluoro-, [bis(1-methylethoxy)phosphinyl]methyl ester](/CAS/20210111/GIF/134963-85-2.gif)
134963-85-2
0 suppliers
inquiry