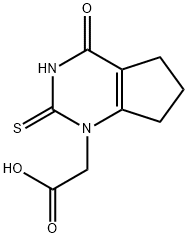
2,3,4,5,6,7-hexahydro-4-oxo-2-thioxo-1H-CyclopentapyriMidine-1-acetic acid synthesis
- Product Name:2,3,4,5,6,7-hexahydro-4-oxo-2-thioxo-1H-CyclopentapyriMidine-1-acetic acid
- CAS Number:1349902-20-0
- Molecular formula:C9H10N2O3S
- Molecular Weight:226.25
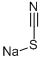
540-72-7
421 suppliers
$13.50/100G

6000-44-8
278 suppliers
$16.96/10gm:
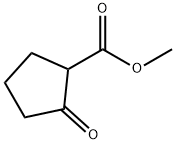
10472-24-9
456 suppliers
$6.00/10g

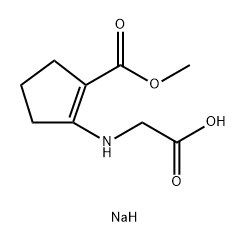
1349902-20-0
6 suppliers
inquiry
Yield:1349902-20-0 58%
Reaction Conditions:
Stage #1: sodium glycinate;methyl 2-oxocyclopentane-1-carboxylatewith 1-methyl-pyrrolidin-2-one at 60; for 2 h;Inert atmosphere;
Stage #2: sodium thiocyanidewith chloro-trimethyl-silane at 120; for 3 h;Inert atmosphere;
Steps:
2-(4-Oxo-2-thioxo-2,3,4,5,6,7-hexahydro-1H-cyclopenta-[d]-pyrimidin-1-yl) acetic acid (1):
Methyl 2-oxocyclopentanecarboxylate (1 eq, 17.5 mmol) was added to a suspension of glycine sodium salt (1 eq, 18.0 mmol) in NMP (18 mL). The reaction mixture was stirred for 2 hours at 60°C under argon. After cooling the mixture to room temperature, sodium thiocyanate (1.4 eq, 25 mmol) was added. Chlorotrimethylsilane (3.5 eq, 61.5 mmol) was then added dropwise and the reaction was stirred at 120° C for 3 hours. The reaction was cooled to 90° C and water (35 mL) was added. The suspension was slowly cooled to 4° C overnight and the product was collected by filtration. The product was washed with water (2 x 15 mL) and acetone (15 mL) then dried at 50° C to yield the title compound as an off white solid (1.4 g, 58%). 1H NMR (DMSO-d6) δ: 12.56 (s, 1H), 4.94 (bs, 2H), 2.85 (m, 2H), 2.59 (t, J = 7.8 Hz, 2H) 1.99 (quint, J = 7.8 Hz, 2H).
References:
Guibbal, Florian;Bénard, Sébastien;Patché, Jessica;Meneyrol, Vincent;Couprie, Jo?l;Yong-Sang, Jennyfer;Meilhac, Olivier;Jestin, Emmanuelle [Bioorganic and Medicinal Chemistry Letters,2018,vol. 28,# 4,p. 787 - 792] Location in patent:supporting information
1349902-19-7
1 suppliers
inquiry
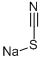
540-72-7
421 suppliers
$13.50/100G

1349902-20-0
6 suppliers
inquiry

10472-24-9
456 suppliers
$6.00/10g

1349902-20-0
6 suppliers
inquiry