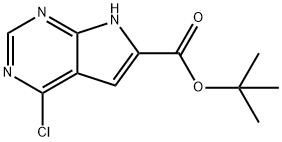
tert-Butyl 4-chloro-7H-pyrrolo[2,3-d]pyrimidine-6-carboxylate synthesis
- Product Name:tert-Butyl 4-chloro-7H-pyrrolo[2,3-d]pyrimidine-6-carboxylate
- CAS Number:1351094-00-2
- Molecular formula:C11H12ClN3O2
- Molecular Weight:253.68
![4-chloro-5-hydroxy-6,7-dihydro-5H-pyrrolo[2,3-d]pyrimidine-6-carboxylic acid tert-butyl ester](/CAS/20200401/GIF/1351093-98-5.gif)
1351093-98-5
1 suppliers
inquiry
![tert-Butyl 4-chloro-7H-pyrrolo[2,3-d]pyrimidine-6-carboxylate](/CAS/20200119/GIF/1351094-00-2.gif)
1351094-00-2
17 suppliers
inquiry
Yield:1351094-00-2 70.4%
Reaction Conditions:
with sodium hydride in N,N-dimethyl-formamide at 0 - 20; for 1 h;
Steps:
I-13 Step 2: 4-Chloro-7H-pyrrolo[2,3-d]pyrimidine-6-carboxylic acid tert-butyl ester
To a solution of tert-butyl 4-chloro-5-hydroxy-6,7-dihydro-5H-pyrrolo[2,3-d]pyrimidine-6- carboxylate (634 mg, 2.33 mmol, Eq: 1.00) in DMF (10 mL) was added sodium hydride (93.3 mg, 2.33 mmol, Eq: 1.00) at 0 °C and then stirred at r.t. for 1 h. The reaction was quenched with water then washed with NH4C1 and brine. The combined organic layers were dried over anhydrous sodium sulfate then the solvent was removed under reduced pressure. The crude material was purified by column chromatography (silica, 5-35% ethyl acetate in hexanes) to give title compound (417 mg, 70.4 % yield) as a white solid. LC/MS: m/z calculated forCnHi2ClN30([M+H]+): 254.6 Found: 254.1
References:
WO2014/64131,2014,A2 Location in patent:Page/Page column 76
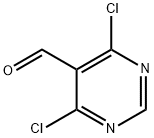
5305-40-8
322 suppliers
$10.00/1g
![tert-Butyl 4-chloro-7H-pyrrolo[2,3-d]pyrimidine-6-carboxylate](/CAS/20200119/GIF/1351094-00-2.gif)
1351094-00-2
17 suppliers
inquiry
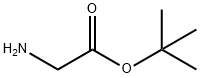
6456-74-2
184 suppliers
$8.00/1g
![tert-Butyl 4-chloro-7H-pyrrolo[2,3-d]pyrimidine-6-carboxylate](/CAS/20200119/GIF/1351094-00-2.gif)
1351094-00-2
17 suppliers
inquiry