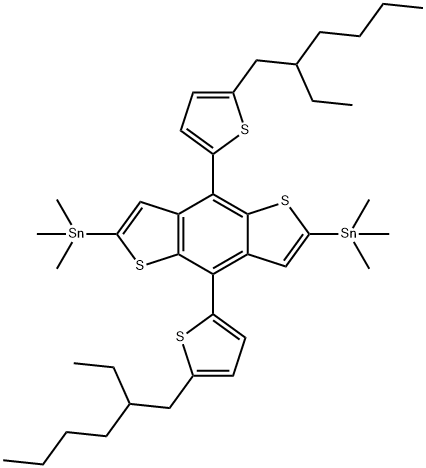
2,6-Bis(triMethyltin)-4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)benzo [1,2-b:4,5-b']dithiophene synthesis
- Product Name:2,6-Bis(triMethyltin)-4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)benzo [1,2-b:4,5-b']dithiophene
- CAS Number:1352642-37-5
- Molecular formula:C40H58S4Sn2
- Molecular Weight:904.57
![4,8-Di(2-(2-ethylhexyl)thiophene-5-yl)-benzo[1,2-b:4,5-b']dithiophene](/CAS/20200611/GIF/1352642-35-3.gif)
1352642-35-3
51 suppliers
$143.00/200mg

1066-45-1
148 suppliers
$26.03/2g
![2,6-Bis(triMethyltin)-4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)benzo [1,2-b:4,5-b']dithiophene](/CAS/20180808/GIF/1352642-37-5.gif)
1352642-37-5
100 suppliers
$45.00/2mg
Yield:1352642-37-5 87%
Reaction Conditions:
Stage #1: 4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)benzo[1,2-b:4,5-b']dithiophenewith n-butyllithium in tetrahydrofuran;hexane at 0 - 20; for 1 h;
Stage #2: trimethylstannyl chloride in tetrahydrofuran;hexane; for 2 h;
Steps:
6.2 (2) Synthesis of Formula F
In tetrahydrofuran (THF) in 100 ml into the compound (2.0 g, 3.44 mmol) of F-1 it lowered the temperature to dissolve back 0 . Insert in the temperature slowly a hexane (hexane) 1.6M n-BuLi (1.6M n-Butyllithium in hexane, 4.36ml, 6.98 mmol) dissolved in the mixture was stirred at room temperature for 1 hour. This solution was dissolved in THF to which 1M trimethyl tin chloride (1MTrimethyltinchloride in THF, 10.3 ml, 10.3 mmol) and stirred for 2 hours was put in a time. Then poured into water to the solution, back extracted twice with diethyl ether (Diethyl ether), washed with water two times, to remove the residue magnesium sulfate (MgSO4) (Magnesiumsulfate). The solvent was removed under reduced pressure and the remaining solution was recrystallized with ethanol (ethanol) to give a solid of a pale yellow crystal. Yield: 87%
References:
KR2015/115974,2015,A Location in patent:Paragraph 0313; 0314; 0318; 0319; 0320
![Benzo[1,2-b:4,5-b']dithiophene-4,8-dione](/CAS/GIF/32281-36-0.gif)
32281-36-0
189 suppliers
$9.00/250mg

4891-44-5
74 suppliers
$21.00/250mg
![2,6-Bis(triMethyltin)-4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)benzo [1,2-b:4,5-b']dithiophene](/CAS/20180808/GIF/1352642-37-5.gif)
1352642-37-5
100 suppliers
$45.00/2mg
18908-66-2
287 suppliers
$5.00/25g
![2,6-Bis(triMethyltin)-4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)benzo [1,2-b:4,5-b']dithiophene](/CAS/20180808/GIF/1352642-37-5.gif)
1352642-37-5
100 suppliers
$45.00/2mg

4891-44-5
74 suppliers
$21.00/250mg
![2,6-Bis(triMethyltin)-4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)benzo [1,2-b:4,5-b']dithiophene](/CAS/20180808/GIF/1352642-37-5.gif)
1352642-37-5
100 suppliers
$45.00/2mg
![Benzo[1,2-b:4,5-b']dithiophene-4,8-dione](/CAS/GIF/32281-36-0.gif)
32281-36-0
189 suppliers
$9.00/250mg
![2,6-Bis(triMethyltin)-4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)benzo [1,2-b:4,5-b']dithiophene](/CAS/20180808/GIF/1352642-37-5.gif)
1352642-37-5
100 suppliers
$45.00/2mg