Benzoic acid, 4-methoxy-2-(1-methylethoxy)-, 1-methylethyl ester synthesis
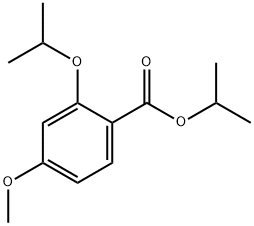
- Product Name:Benzoic acid, 4-methoxy-2-(1-methylethoxy)-, 1-methylethyl ester
- CAS Number:1353891-99-2
- Molecular formula:C14H20O4
- Molecular Weight:252.31
Yield:1353891-99-2 70%
Reaction Conditions:
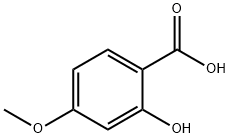
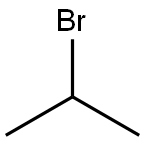
Stage #1: 4-methoxysalicylic acid;isopropyl bromidewith potassium carbonate in N,N-dimethyl-formamide; for 31 h;Reflux;
Stage #2: with potassium iodide in N,N-dimethyl-formamide; for 17 h;
Steps:
2
isopropyl 2-isopropoxy-4-methoxybenzoate. See Hattori et al., 2003, which is incorporated herein by reference. Isopropyl bromide (2.926 g, 23.79 mmol) was added to a stirred mixture of 4-methoxysalicylic acid (1.000 g, 5.947 mmol), K2CO3 (3.286 g, 23.79 mmol), and dry DMF (30 mL) at room temperature. The mixture was allowed to stir for 20 min before heating to reflux for 31 h. The reaction mixture was cooled to room temperature, treated with KI (98.7 mg, 595 μmol) and stirred for 17 h. The reaction was quenched with 1 M aq HCl then extracted with diethyl ether. The combined organic layers were washed with 1 M aq Na2CO3, water, then brine before drying over MgSO4. Concentration of the dried organic layers resulted in a bronze oil (1.0549 g, 70%) that was pure by 1H NMR. Rf=0.16 (5% EtOAc/hexanes); IR (film) 2979, 2936, 1721, 1694, 1608, 1575 cm-1; 1H NMR (400 MHz, CDCl3) δ 7.77 (d, J=7.2 Hz, 1H), 6.49-6.43 (m, 2H), 5.20 (heptet, J=6.4 Hz, 1H), 4.55 (heptet, J=6.0 Hz, 1H), 3.81 (s, 3H), 1.36 (d, J=6.0 Hz, 6H), 1.33 (d, J=6.0 Hz, 6H); 13C NMR (100 MHz, CDCl3) ppm 165.8, 163.5, 159.3, 133.4, 114.9, 104.9, 101.8, 71.5, 67.5, 55.4, 22.0 (2C); HRMS (CI): Exact mass calcd for C14H21O4[M+H]+ 253.1434. found 253.1431.
References:
US2012/88915,2012,A1 Location in patent:Page/Page column 13