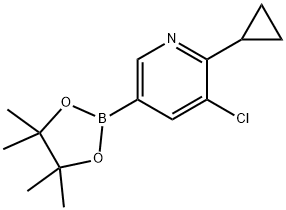
3-chloro-2-cyclopropyl-5-(tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine synthesis
- Product Name:3-chloro-2-cyclopropyl-5-(tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine
- CAS Number:1355067-20-7
- Molecular formula:C14H19BClNO2
- Molecular Weight:279.57
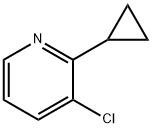
1355066-87-3
17 suppliers
$130.00/100mg

73183-34-3
575 suppliers
$6.00/5g

1355067-20-7
14 suppliers
$65.00/10mg
Yield:1355067-20-7 86%
Reaction Conditions:
(1,5-cyclooctadiene)(methoxy)iridium(I) dimer;4,4'-di-tert-butyl-2,2'-bipyridine in n-heptane at 20; for 18 h;Inert atmosphere;
Steps:
69
A round bottom flask was charged with 3-chloro-2-cyclopropylpyridine (Preparation 48, 0.475 g; 3.092 mmol), bis(pinacolato)diboron (0.980 g, 3.859 mol) and 4,4-di-tert-butyl-2,2-dipyridyl (0.025 g; 0.093 mmol) in heptane (1.55 L). The reaction mixture was cycled between vacuum and nitrogen 6 times over 15 minutes. Di-methanolatodiiridium(Ir-Ir)-cycloocta-1,5-diene (1:2) (0.063 g; 0.093 mmol) was then added and the reaction stirred for 18 hours under nitrogen atmosphere at room temperature. The reaction mixture was evaporated to dryness to afford a red viscous oil. The resulting oil was dissolved in acetone (10.0 mL) and cooled to 0° C. with an ice bath. Then potassium peroxymonosulfate (2.55 g, 4.15 mmol) in water (10.0 mL) was added dropwise to the mixture and stirred at this temperature for 1 hour. The reaction was then diluted in tert-butyl methyl ether (25.0 mL) and washed with brine (3×25.0 mL). The organic layer was then dried over sodium sulfate, filtered, and concentrated in vacuo. The resulting crude product was purified with silica gel chromatography eluting with 0 to 30% EtOAc in heptane to yield the title compound as a pale yellow solid (0.220 g, 1.28 mmol, 42%):1H NMR (400 MHz, d6-DMSO): δ 0.81-0.85 (m, 2H), 0.86-0.91 (m, 2H), 2.26-2.32 (m, 1H), 7.19 (d, 1H), 7.94-7.95 (d, 1H), 10.05 (s, 1H).LCMS Rt=1.85 minutes MS m/z 170 [MH]+
References:
US2012/10183,2012,A1 Location in patent:Page/Page column 57
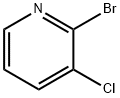
96424-68-9
205 suppliers
$9.00/5g

1355067-20-7
14 suppliers
$65.00/10mg