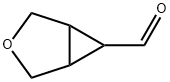
(1R,5S,6S)-Rel-3-oxabicyclo[3.1.0]hexane-6-carbaldehyde synthesis
- Product Name:(1R,5S,6S)-Rel-3-oxabicyclo[3.1.0]hexane-6-carbaldehyde
- CAS Number:135576-99-7
- Molecular formula:C6H8O2
- Molecular Weight:112.1265
![trans-3-Oxabicyclo[3.1.0]hexane-6-Methanol](/CAS/20150408/GIF/135577-15-0.gif)
135577-15-0
24 suppliers
$75.00/10g
![(1R,5S,6S)-Rel-3-oxabicyclo[3.1.0]hexane-6-carbaldehyde](/CAS/20180808/GIF/135576-99-7.gif)
135576-99-7
15 suppliers
$195.00/100MG
Yield:135576-99-7 2.71 g
Reaction Conditions:
Stage #1: (1R,5S,6R)-3-oxabicyclo[3.1.0]hexane-6-ylmethanolwith oxalyl dichloride;dimethyl sulfoxide in dichloromethane at -78; for 1 h;
Stage #2: with triethylamine in dichloromethane; for 0.5 h;
Steps:
To a solution of DMSO (4.82 mL, 67.9 mmol) in CH2Cl2 (70 mL) was added oxalyl chloride (3.14 mL, 35.8 mmol) at -78 °C dropwise slowly. The resulting mixture was stirred at -78 °C for 15 min. A solution of Cap-1 step b (3.10 g, 27.2 mmol) in 35 mL of CH2CI2 was added and the mixture was stirred at -78 °C for 1 h. Et3N (18.93 mL, 136 mmol) was then added dropwise. After 30 min, the cooling bath was removed and the reaction was quenched with cold 20% K2HPO4 aq. solution (10 mL) and water. The mixture was stirred at room temperature for 15 min then diluted with Et2O. The layers were separated. The aqueous layer was extracted with Et2O (2X). The combined organic layers were washed with brine, dried with MgSO4 and concentrated. The residue was purified by flash chromatography (silica gel, 100% CH2Cl2) to afford Cap-1 step c (2.71 g) as light yellow oil.1H NMR (500 MHz, CDCl3) δ ppm 9.41 (1 H, d, J=4.27 Hz), 3.96 (2 H, d, J=8.85 Hz), 3.80 (2 H, d, J=8.55 Hz), 2.27 - 2.33 (2 H, m), 1.93 (1 H, m)
References:
WO2014/65791,2014,A1 Location in patent:Page/Page column 40
![3-Oxabicyclo[3.1.0]hexane-6-carboxylic acid, 1,1-dimethylethyl ester, (1α,5α,6α)-](/CAS/20211123/GIF/1307304-62-6.gif)
1307304-62-6
0 suppliers
inquiry
![(1R,5S,6S)-Rel-3-oxabicyclo[3.1.0]hexane-6-carbaldehyde](/CAS/20180808/GIF/135576-99-7.gif)
135576-99-7
15 suppliers
$195.00/100MG