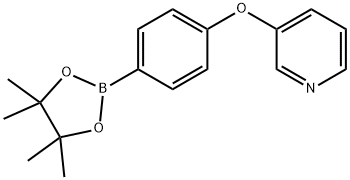
3-[4-(4,4,5,5-Tetramethyl-[1,3,2]Dioxaborolan-2-Yl)-Phenoxy]-Pyridine(WX652036) synthesis
- Product Name:3-[4-(4,4,5,5-Tetramethyl-[1,3,2]Dioxaborolan-2-Yl)-Phenoxy]-Pyridine(WX652036)
- CAS Number:1362703-30-7
- Molecular formula:C17H20BNO3
- Molecular Weight:297.16
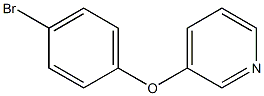
18085-61-5
5 suppliers
inquiry

73183-34-3
563 suppliers
$6.00/5g
![3-[4-(4,4,5,5-Tetramethyl-[1,3,2]Dioxaborolan-2-Yl)-Phenoxy]-Pyridine(WX652036)](/CAS/20200611/GIF/1362703-30-7.gif)
1362703-30-7
21 suppliers
$534.00/1g
Yield:1362703-30-7 61 %
Reaction Conditions:
Stage #1: 3-(4-bromophenoxy)pyridine;bis(pinacol)diboranewith potassium acetate in 1,4-dioxane;N,N-dimethyl-formamide;Inert atmosphere;
Stage #2: with (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride in 1,4-dioxane;N,N-dimethyl-formamide at 95;Inert atmosphere;
Steps:
Synthesis towards molecular precursor 1
A mixture of 3-(4-bromophenoxy)pyridine (1.05 g, 4.20 mmol,1.0 equiv.), bis(pinacolato)diboron (2.35 g, 9.24 mmol, 2.2 equiv.) and KOAc (1.24 g, 12.6 mmol, 3.0 equiv.) in dry dioxane (5.3 ml) and dry DMF (0.7 ml) was degassed under nitrogen for 45 min. Subsequently, Pd(dppf)Cl2 (92.2 mg, 126 μmol, 0.03 equiv.) was added and the mixture was stirred for 62 h at 95 °C. After cooling down to room temperature, the crude mixture was filtered over Celite and concentrated under reduced pressure. The residue was purified by flash chromatography(SiO2, dichloromethane to dichloromethane/ethyl acetate 90:10).3-(4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenoxy)pyridine.(1). Yield: 766 mg, 2.58 mmol (61%, Lit. 63: not reported). 1H NMR(400 MHz, CDCl3, r.t.): δ = 8.44-8.42 (m, 1H, H2), 8.39 (dd, 3JH-H = 4.5 Hz,4JH-H = 1.6 Hz, 1H, H6), 7.83-7.79 (m, 2H, H3′), 7.33-7.27 (m, 2H, H4 & H5),7.02-6.98 (m, 2H, H2′), 1.35 (s, 12H) ppm. Analytical data are in accordance with the literature63.
References:
Noll, Niklas;Krause, Ana-Maria;Beuerle, Florian;Würthner, Frank [Nature Catalysis,2022,vol. 5,# 10,p. 867 - 877]
80650-45-9
63 suppliers
$31.00/200μmol
![3-[4-(4,4,5,5-Tetramethyl-[1,3,2]Dioxaborolan-2-Yl)-Phenoxy]-Pyridine(WX652036)](/CAS/20200611/GIF/1362703-30-7.gif)
1362703-30-7
21 suppliers
$534.00/1g