
5-(cyclobutylethynyl)-3-fluoro-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole synthesis
- Product Name:5-(cyclobutylethynyl)-3-fluoro-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole
- CAS Number:1365889-99-1
- Molecular formula:C18H19FN2O
- Molecular Weight:298.35

1268810-13-4
16 suppliers
$750.00/1g
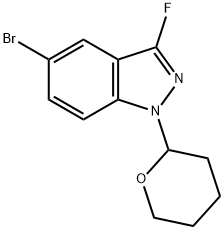
1365889-96-8
17 suppliers
$1676.00/1g

1365889-99-1
17 suppliers
inquiry
Yield:1365889-99-1 7.4 g
Reaction Conditions:
with 1,1'-bis-(diphenylphosphino)ferrocene;copper(l) iodide;palladium diacetate;caesium carbonate in N,N-dimethyl acetamide at 80; for 10 h;Inert atmosphere;
Steps:
5-(Cyclobutylethynyl)-3-fluoro-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole
Nitrogen was bubbled through a solution of 5-bromo-3 -fluoro-1 -(tetrahydro-2H- pyran-2-yl)-1H-indazole (11 g, 36.8 mmol, Intermediate 1) and DMA (40 mL) for 5 mm. With continued N2 bubbling, CuT (699 mg, 3.68 mmol), Pd(OAc)2 (826 mg, 3.68 mmol), dppf (2.04 g, 3.68 mmol), Cs2CO3 (16.8 g, 51.5 mmol), and (cyclobutylethynyl)trimethylsilane (7.3 g, 47.8 mmol, Intermediate 13) were added in sequence. The reaction mixture was heated at 80 °C under N2 for 10 h, diluted with ethyl acetate (400 mL) and water (200 mL), and then filtered. The aqueous layer was extracted with ethyl acetate (50 mL x 2), and the organic extracts were combined with organic layer of the filtrate. The combined organics were washed with brine (100 mL x 3), dried (Na2SO4), filtered, concentrated, and purified by silica gel chromatography (1:70; ethyl acetate:petroleum ether) to give 7.4 g of 5-(cyclobutylethynyl)-3-fluoro-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole as a yellow solid. 1H NMR (400 MHz; DMSO-d6): ? 7.77 (s, 1H), 7.73 (d, 1H), 7.49 (dd, 1H), 5.80-5.78 (m, 1H), 3.88-3.86 (m, 1H), 3.75-3.68 (m, 1H), 3.3 1-3.25 (m, 1H), 2.35-2.27 (m, 2H), 2.24-2.10 (m, 3H), 2.01-1.85 (m, 4H), 1.74-1.65 (m, 1H), 1.57-1.51 (m, 2H); LCMS: 299 (M+H).
References:
WO2013/142266,2013,A1 Location in patent:Paragraph 00514
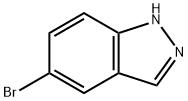
53857-57-1
362 suppliers
$6.00/1g

1365889-99-1
17 suppliers
inquiry
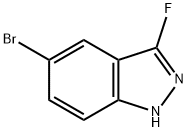
1211537-09-5
45 suppliers
$45.00/2.5mg

1365889-99-1
17 suppliers
inquiry
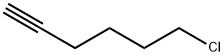
10297-06-0
92 suppliers
$22.00/1g

1365889-99-1
17 suppliers
inquiry

113964-33-3
17 suppliers
inquiry

1365889-99-1
17 suppliers
inquiry